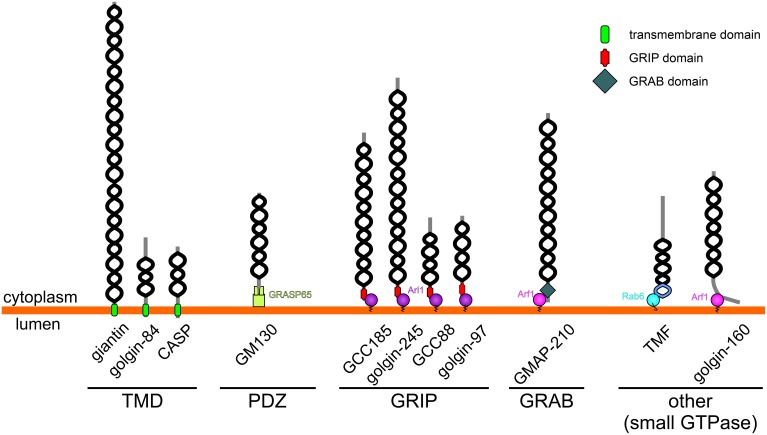
Figure 1.
Topology and membrane attachment of golgins. Golgins are comprised predominantly of coiled-coil regions (depicted by black helices; note that the frequent short breaks found within the coiled-coil regions of golgins are not shown for simplicity). Golgins can either be directly anchored to the Golgi membrane through carboxy-terminal transmembrane domains (giantin, golgin-84 and CASP), or they can associate with the Golgi membrane through interactions with other Golgi-localized proteins. The carboxy-terminus of GM130 acts as a ligand for the PDZ domains of GRASP65. Trans-Golgi golgins have a GRIP domain that interacts with Arl1, whereas GMAP-210 has an analogous GRAB domain that binds to Arf1. TMF and golgin-160 are attached to the Golgi membrane via binding of their carboxy-terminal regions to Rab6 or Arf1 respectively.