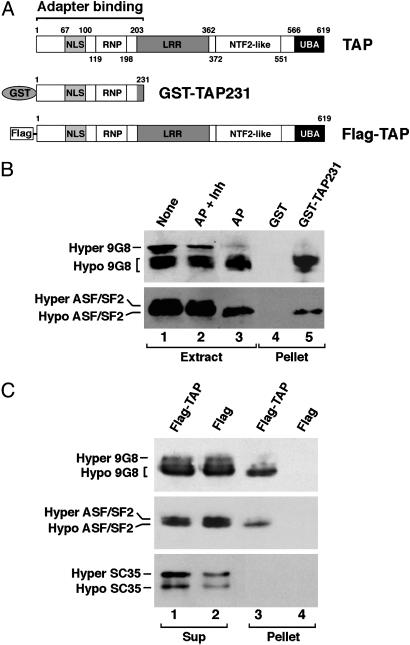
Fig. 1.
TAP preferentially binds hypophosphorylated 9G8 and ASF/SF2. (A) Schematic of the domain structures of TAP (2, 29) and the recombinant proteins used in the binding assays. (B) TAP binding to 9G8 and ASF/SF2 in vitro. Bacterially expressed and affinity-purified GST-TAP231 (lane 5) or GST alone (lane 4) prebound on glutathione beads was incubated with HeLa cell nuclear extract. Bound (Pellet) fractions were resolved by 10% SDS/PAGE, followed by Western blot analysis by using anti-9G8 (Upper) or anti-ASF/SF2 (Lower) antibodies. Parallel extract samples not treated (None, lane 1), treated with alkaline phosphatase plus phosphatase inhibitors (AP + Inh, lane 2), or treated with AP (AP, lane 3) indicate the positions of differentially phosphorylated forms of 9G8 (Upper) and ASF/SF2 (Lower). (C) TAP binding to 9G8 and ASF/SF2 in vivo. HEK293 cells were transiently transfected with plasmids pcDNA-Flag-TAP expressing a Flag-tagged full-length TAP or pcDNA-Flag alone. The Flag-TAP or Flag was immunoprecipitated from the transfected cell extracts with anti-Flag antibodies. Five percent of the supernatants (Sup, lanes 1 and 2) and the precipitated proteins (Pellet, lanes 3 and 4) were analyzed by Western blotting by using anti-9G8 (Top), anti-ASF/SF2 (Middle), or anti-SC35 (Bottom) antibodies.