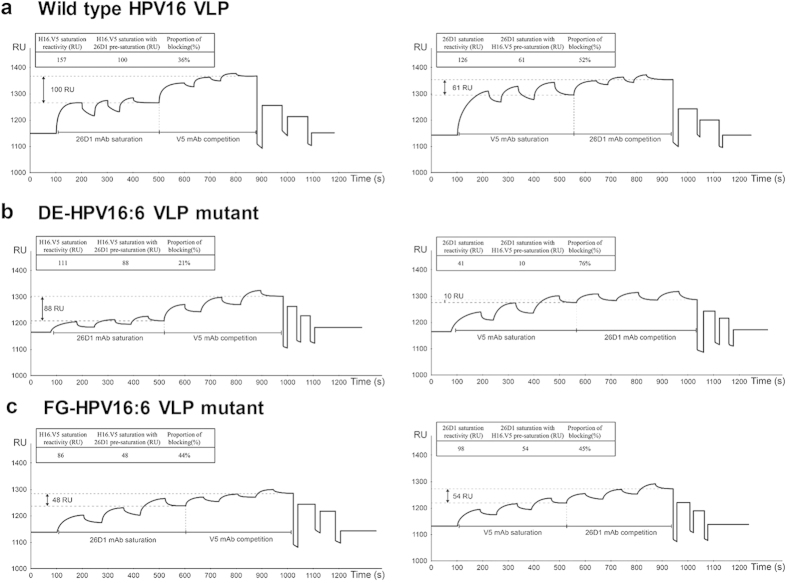
Figure 5. Surface plasmon resonance of a pairwise binding study for cross inhibition between 26D1 and H16.V5.
(a) Epitope competition between 26D1 and H16.V5 on binding to wild type HPV16 VLPs which were covalently attached to a sensor chip. The saturation units for 26D1 and H16.V5 (100 μg/ml, injected three times) were 126 RU and 157 RU, respectively. Epitope competition was established by pre-saturation of the surface by 26D1 followed by injecting a saturating amount of H16.V5. With pre-saturation of 26D1, the H16.V5 saturation level was 100 RU, corresponding to a 36% reduction of the original H16.V5 saturation binding (left). We reciprocally assessed 26D1 saturated binding level with H16.V5 pre-saturation, which resulted in 52% reduction of the original 26D1 saturated level (right). (b) Epitope competition between 26D1 and H16.V5 on binding to DE-HPV16:6 VLP mutant (HPV16 VLPs with DE loops swapped with the type 6 loops). The saturation units for 26D1 and H16.V5 were 41 RU and 111 RU, respectively. 21% of H16.V5 binding epitopes were inhibited with 26D1 pre-saturation (left), and 76% of 26D1 binding epitopes were blocked by H16.V5 pre-saturation (right). (c) Epitope competition between 26D1 and H16.V5 on binding to FG-HPV16:6 VLP mutant (HPV16 VLPs with FG loops swapped with the type 6 loops). The saturation units for 26D1 and H16.V5 were 98 RU and 86 RU, respectively. After 26D1 pre-saturation, the saturated binding level for H16.V5 was 48 RU and resulted in 44% reduction of the H16.V5 saturation binding (left). Reciprocally, 45% of 26D1 binding epitopes were blocked by H16.V5 pre-saturation (right). The saturation resonance units (RU) for mAbs with or without pre-saturation and blocking proportion are indicated in the figure.