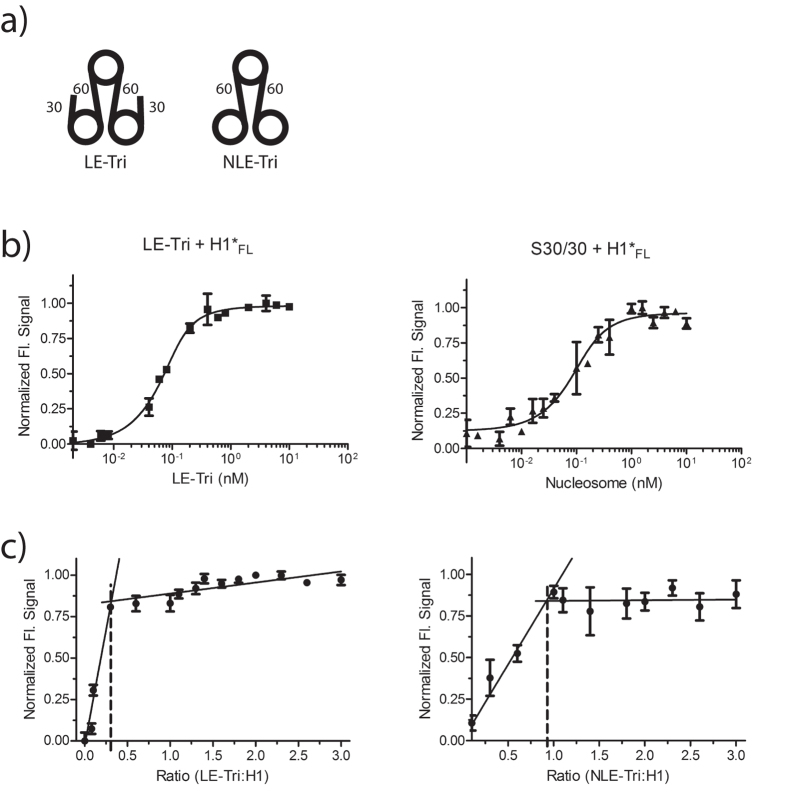
Figure 3. Neighboring nucleosomes do not contribute to H1 binding.
(a) Schematic of trinucleosome constructs. The constructs differ only in the amount of DNA extending from the two terminal nucleosomes. (b) Representative (de)quenching curve of LE-Tri, and S30/30 with H1FL. H1 was held constant at 0.1 nM and trinucleosome was titrated (0–20 nM respectively); curves were fit with a quadratic equation (Eq. 3). Kd values obtained for S30/30 are identical within error of values obtained by FRET (Table 1). (c) Stoichiometry of H1FL complexes with trinucleosomes (LE-Tri; left, and NLE-Tri; right). Trinucleosomes were titrated (0.8–30nM) into a constant amount of H1 (10nM). For LE-Tri, we find a molar ratio of 0.3 LE-Tri to one H1 (or 1 H1 per nucleosome). For NLE-Tri we observe a stoichiometry of ~0.9 NLE-Tri per H1 (or one H1 per trimer).