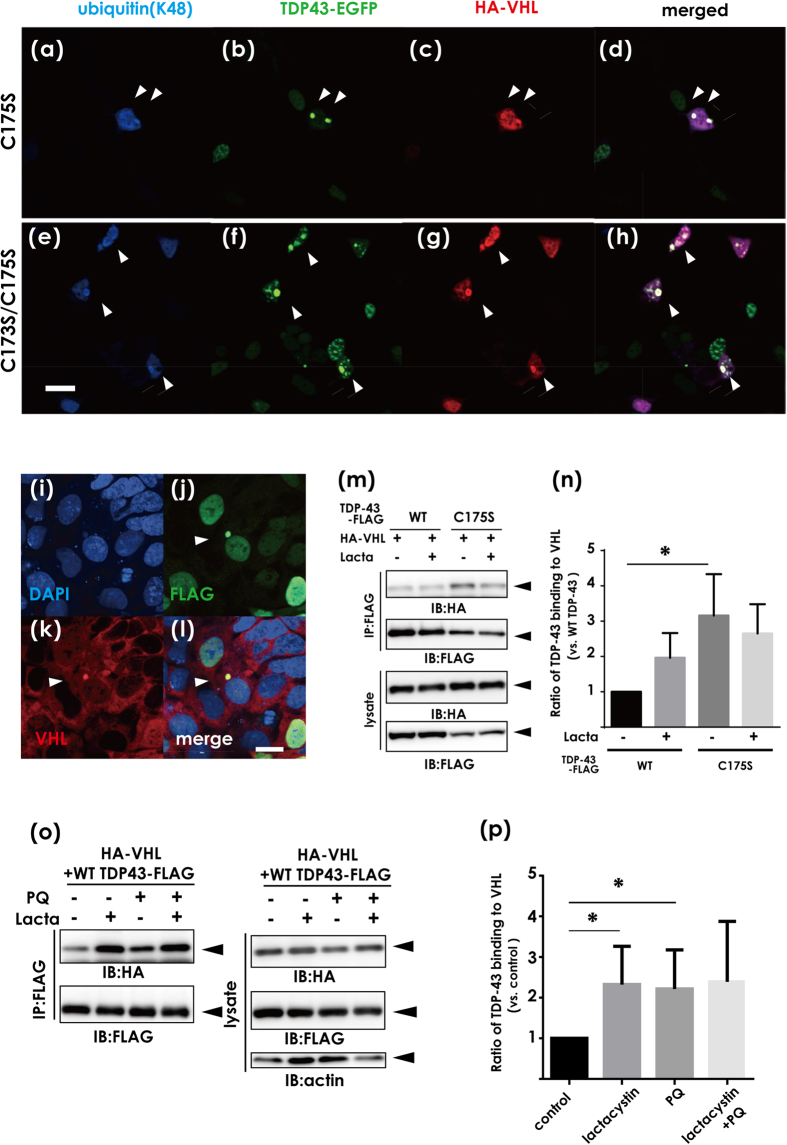
Figure 2. Effects of TDP-43 conformation on the interaction with the VHL/CUL2 complex.
(a–h) Confocal laser micrographs of HEK293A cells expressing HA-VHL and aggregation-prone mutants (C173S and/or C175S). (a–d) for C175S and (e–h) for C173S/C175S. Arrowheads indicate co-localization of TDP-43 aggregates of ubiquitin and VHL. Scale bar, 50 μm. (i–l) Confocal laser micrographs showing that TDP-43 aggregates colocalize with endogenous VHL in HEK293A cells. HEK293A cells were fixed and assayed by immunofluorescence with antibodies against FLAG (green) and VHL (red). Scale bar, 20 μm. (m,n) The lysates from HEK293A cells were transfected with TDP-43-FLAG (WT or C175S) and HA-VHL, and the cell lysates were immunoprecipitated with anti-FLAG and analyzed by Western blotting using anti-HA or anti-FLAG antibodies (m), and by the densitometric analysis (n). Aggregation-prone mutants showed a significantly higher affinity with VHL than WT. Differences were evaluated by one-way ANOVA (mean ± SD from triplicates; *p < 0.05 versus WT). (o) Western blotting showing the effect of oxidative stress induced by paraquat on the binding affinity of TDP-43 to VHL. HEK293A cells transfected with HA-VHL and WT TDP-43-FLAG were treated with lactacystin (10 μM, 8 h) and/or paraquat (PQ, 0.4 mM, 8 h). (p) Bands detected by the anti-HA antibody in (n) were assessed by densitometry. Each data point was obtained by normalization to TDP-43 and is expressed as the average of three independent experiments. Differences were evaluated by one-way ANOVA (mean ± SD from triplicates; *p < 0.05 versus control).