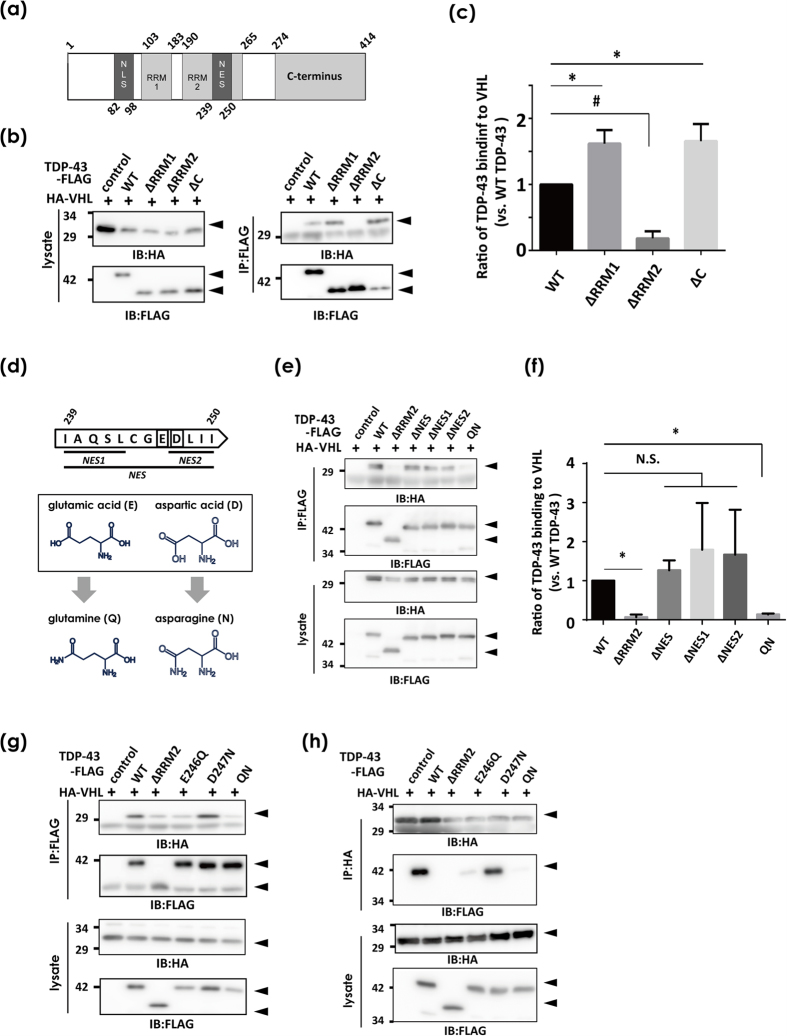
Figure 3. VHL recognition site in TDP-43.
In vivo pull-down assay of HEK293A cells transfected with HA-VHL and WT TDP-43-FLAG devoid of various domains. (a) Schematic showing of the domain profiles of FL TDP-43. (b,c) RRM2 domain is required for the interaction between TDP-43 and VHL. Total lysates in co-IP buffer were immunoprecipitated with anti-FLAG affinity beads to pull-down TDP-43-FLAG devoid of RRM1 (∆RRM1), RRM2(∆RRM2), or C-terminus (∆C), and the bound HA-VHL was analyzed by Western blotting using anti-HA and anti-FLAG antibodies (b) and quantified by the densitometry (c). Differences were evaluated by one-way ANOVA (mean ± SD from triplicates; *or #p < 0.05 versus WT). (d–h) Identification of E246 in RRM2 as a crucial sequence for recognition by VHL. (d) Scheme showing the subdomains in nucleus exporting signal (NES) subdomains (residues 239–250 in human TDP-43). (e,f ) WT TDP-43-FLAG, ∆RRM1, ∆RRM2, ∆NES, ∆NES1, ∆NES2, or E246Q/D247N mutants (QN), together with HA-VHL were transfected into HEK293A cells. Cell lysates were immunoprecipitated with anti-FLAG and immunoblotted with anti-HA antibody. Differences were evaluated by one-way ANOVA (mean ± SD from triplicates; *or #p < 0.05 versus WT). (g,h) The effect of a single substitution mutant of TDP-43 was similarly analyzed. WT, ∆RRM2, E246Q, D247N, and QN mutants were used. E246Q showed lower affinity to VHL, but D247N did not. (e) for IP-FLAG and HA blot, (f ) IP-HA and FLAG blot.