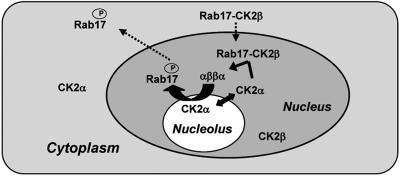
Fig. 6.
Model for nucleo/cytoplasmic trafficking of Rab17. Because mRab17 is retained in the nucleolus, whereas Rab17 is efficiently removed from this organelle, phosphorylation of Rab17 could occur in the nucleus. In this context, the interaction between CK2β and Rab17 can be of significant physiological relevance for nuclear targeting. We propose that after translation in the cytoplasm, Rab17 may interact with CK2β, and, in this way, the Rab17/CK2β complex formed would travel to the nucleus where the three catalytic subunits of CK2 are predominantly located. In the nucleus, CK2α would disrupt Rab17/CK2β to associate with CK2β, generating the holoenzyme, and Rab17 would probably be efficiently phosphorylated by CK2. Once phosphorylated, Rab17 would go to the nucleoplasm and/or cytoplasm to exert its still-unknown function.