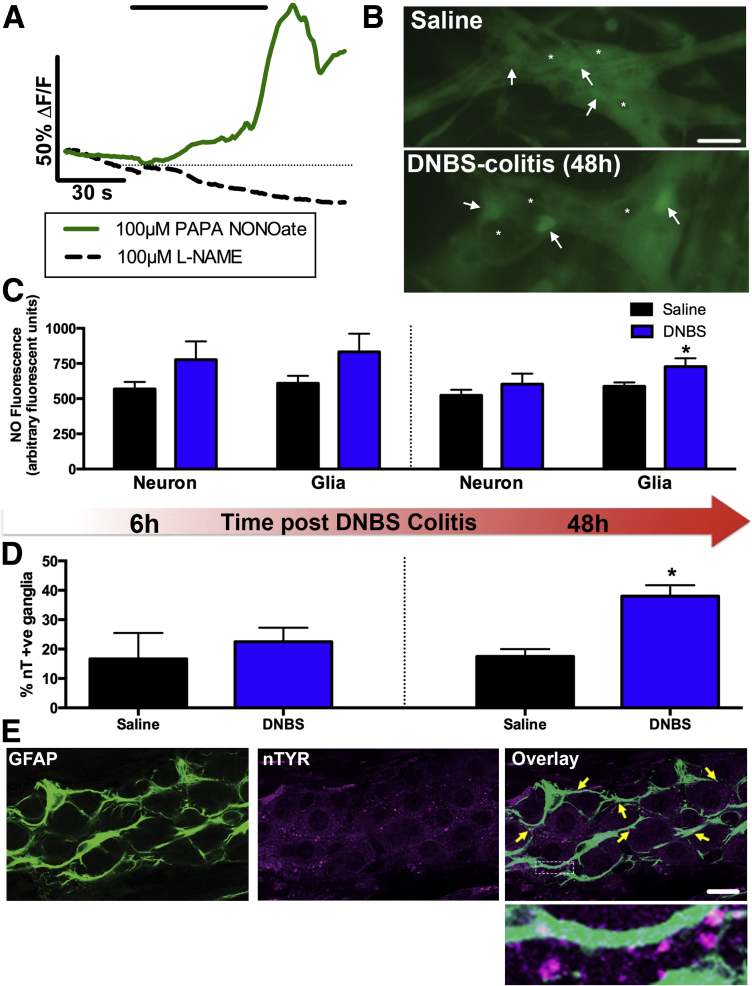
Figure 5.
Enteric glia contribute to oxidative stress by producing nitric oxide (NO) during inflammation. (A–C) In situ NO imaging with the NO sensitive dye 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM). (A) Representative traces of mean glial NO responses after treatment with the NO donor propylamine propylamine NONOate (PAPA NONOate, solid green line, 100 μM) or the pan-nitric oxide synthase (NOS) inhibitor Nω-nitro-l-arginine methyl ester (L-NAME, dashed black line, 100 μM). (B) Representative images of DAF-FM fluorescence in myenteric ganglia from healthy (saline) or inflamed (dinitrobenzene sulfonic acid [DNBS] colitis) mice. Arrows point to representative glial cells and representative neurons (or the lack thereof) are denoted by asterisks (scale bar: 30 μM). (C) Quantification of DAF-FM fluorescence in observable myenteric neurons and glia in the healthy (saline) or inflamed colon at 6 hours (left) and 48 hours (right) after the initiation of DNBS-colitis (n = 5–10 animals; *P < .05, t test compared to glia-saline). (D) Percentage of nitrotyrosine immunoreactive (nT + ve) ganglia in the myenteric plexus of saline and DNBS-treated animals at 6 hours (left) and 48 hours (right) after the initiation of DNBS-colitis (n = 3–5 animals; *P < .05, unpaired t test). (E) Representative myenteric ganglion showing immunoreactivity for nitrated proteins (nTYR; magenta). Enteric glia are labeled with the glial cell marker GFAP (green) and yellow arrowheads highlight areas of colocalization (scale bar: 20 μM). The boxed region in the overlay image is shown at a higher magnification to highlight immunoreactivity of nitrated proteins on glial processes.