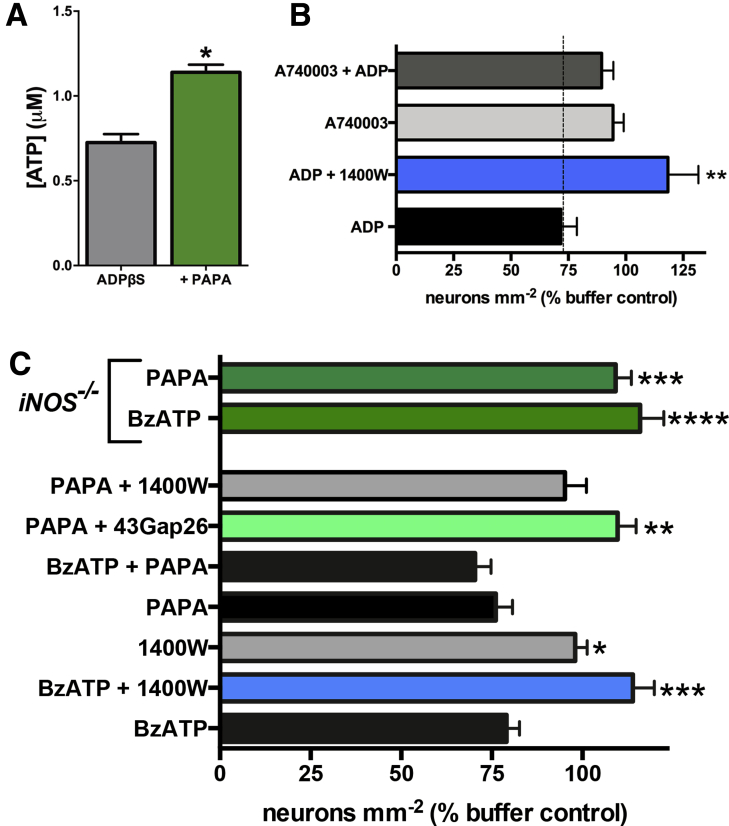
Figure 6.
Nitric oxide (NO) potentiates adenosine triphosphate (ATP) release from glia and promotes neuron death in situ through a mechanism that involves glial connexin-43 (Cx43) hemichannels. (A) ATP release from myenteric ganglia after stimulation of glial P2Y1 receptors (P2Y1Rs) with adenosine 5′-[β-thio]diphosphate trilithium salt (ADPβS, 100 μM) alone or in the presence of the NO donor propylamine propylamine NONOate (PAPA NONOate, n = 4; *P < .05, unpaired t test). (B) Mean packing density of HuC/D-immunoreactive myenteric neurons after direct glial P2Y1R stimulation with adenosine diphosphate (ADP) and inhibition of inducible nitric oxide synthase (iNOS, 1400W; 10 μM) or P2X7 receptors (P2X7Rs, A740003; 10 μM). Inhibition of iNOS or P2X7Rs protects against P2Y1R-driven neuron death (*P ≤ .01, analysis of variance [ANOVA] as compared to ADP; n = 3–4 animals). (C) Mean packing density of myenteric neurons after application of BzATP or NO-modifying drugs in wild-type (bottom bars) or iNOS-knockout mice (iNOS−/−, top two green bars). Inhibition (1400W; 10 μM) or ablation (iNOS−/−) of iNOS protects against P2X7R-driven neuron death. The NO donor PAPA NONOate (100 μM) drives neuron death to an equal extent as BzATP but the combination is not additive. Like BzATP, PAPA NONOate–driven neuron death requires iNOS (blocked by 1400W and in iNOS−/− mice) and Cx43 hemichannel opening (blocked by 43Gap26). *P ≤ .05, **P ≤ .01, ***P ≤ .001, ****P ≤ .0001, ANOVA as compared to BzATP; n = 3–11 animals.