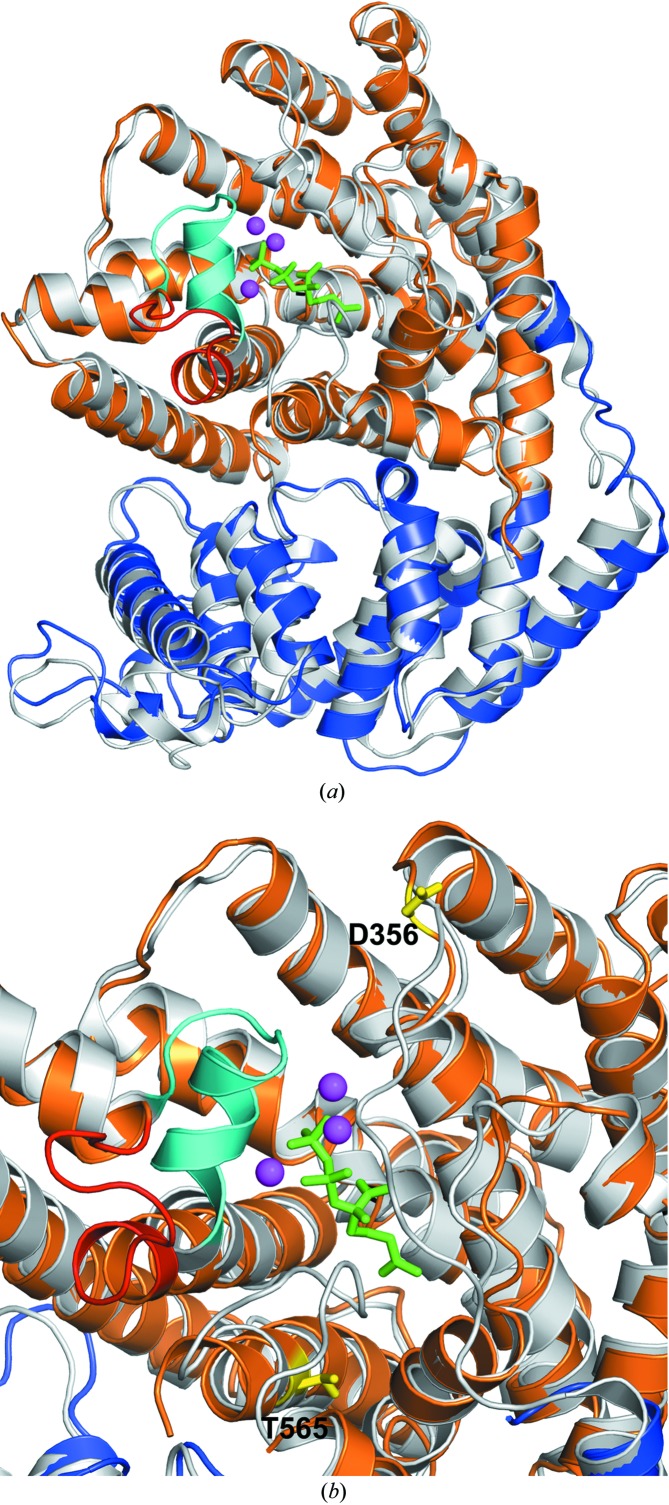
Figure 6.
Structural superposition of TvTPS (monomer B; N-terminal domain coloured blue, C-terminal domain coloured orange) with the crystal structure of ligand-bound (4S)-limonene synthase (coloured light grey; PDB entry 2ong, monomer A). (a) The structural alignment reveals the putative active centre of TvTPS, which is located near the binding site of the substrate analogue 2-fluorogeranyl diphosphate (shown in green sticks), and three coordinated Mn2+ ions (magenta spheres) bound to (4S)-limonene synthase. In proximity, a loop (coloured in cyan) covers the active site of ligand-bound (4S)-limonene synthase. In ligand-free TvTPS1 this loop shows a different conformation (coloured red). (b) Close-up view of the active-site regions of TvTPS1 and (4S)-limonene synthase. In addition, the side chains of residues Asp356 and Thr565, which were mutated in this study, are shown as yellow sticks and labelled.