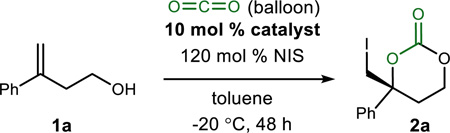
Table 1.
Development of an Enantioselective CO2-Capture Reaction using a Homoallylic Alcohol
 | |||||
|---|---|---|---|---|---|
| entry | ligand (base) | acida | notesb | yieldc | ee (%)d |
| 1 | none | – | – | 0% | – |
| 2 | none | – | NaHe | 16% | – |
| 3 | none | – | NaH (THF)e | 25% | – |
| 4 | DMAP (5)f | – | – | trace | – |
| 5 | DBU (6)f | – | – | trace | – |
| 6 | TBD (4)f | – | – | 11% | – |
| 7 | TFAf | – | – | 6% | – |
| 8 | 3f | – | – | 4% | – |
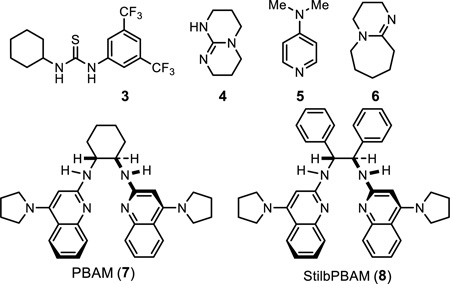
| 9 | PBAM (7) | – | – | 18% | 39 |
| 10 | StilbPBAM (8) | – | – | 33% | 36 |
| 11 | 8 | – | MS | 35% | 60 |
| 12 | 8 | HOTf | MS | 62% | 74 |
| 13 | 8 | H2NTf | MS | 52% | 62 |
| 14 | 8 | F6C3(SO2)2NH | MS | 70% | 86 |
| 15 | 8g | HNTf2 | MS, 0.4 M | 95% | 91 |
| 16 | 8 | HNTf2 | MS, 0.2 M | 51% | 89 |
| 17 | 8 | HNTf2 | MS, 0.1 M | 10% | 79 |
| 18 | 5f | – | – | trace | – |
| 19 | 4f | – | MS | 30% | – |
| 20 | 6f | – | MS | 13% | – |
| 21 | 6f | ½ HNTf2 | MS | 4% | – |
Catalyst prepared as the 1:1 acid salt, except entry 21.
MS denotes molecular sieves 4A, employed at a concentration of 1 g/mmol relative to the alcohol.
Isolated yield.
Enantiomeric excess (ee) determined by HPLC using a chiral stationary phase. Reactions are 0.4 M toluene unless otherwise noted.
Reaction temp = 0 °C. 3-Methyl-3-buten-1-ol was converted in 60% yield under identical conditions.
20 mol % catalyst employed.