Abstract
Background:
Fixed combination calcipotriol 50 µg/g (Cal; as hydrate) plus betamethasone 0.5 mg/g (as dipropionate; BD) has been formulated in an innovative aerosol foam.
Objective:
To assess systemic safety of Cal/BD aerosol foam.
Methods:
In a multicentre, single-arm, open-label, maximal-use systemic-exposure trial, adult patients with moderate to severe, extensive psoriasis (15%-30% of body surface area, including ≥30% of scalp) applied Cal/BD foam once daily. Endpoints were week 4 abnormal adrenocorticotropic hormone (ACTH) challenge test and change in albumin-corrected serum calcium, 24-hour urinary calcium excretion, and urinary calcium-creatinine ratio.
Results:
35 patients reaching week 4 exhibited normal ACTH responses. At week 4, changes in calcium homeostasis were minor and not clinically relevant; no patients experienced elevations above normal. Disease severity generally improved, and 49% of patients achieved treatment success according to the Physician’s Global Assessment of Disease Severity.
Conclusion:
No clinically relevant HPA axis or calcium homeostasis impact was observed with 4 weeks of once-daily Cal/BD foam in patients with extensive psoriasis vulgaris.
Keywords: dermatology, psoriasis
Psoriasis is a chronic, inflammatory disease that affects 2% to 4% of Western populations and manifests as red, scaly plaques on the skin.1 A recent World Health Organization resolution aims to bring attention to the condition, describing it as a disfiguring and disabling disease for which there is no cure.2 Psoriasis is frequently accompanied by multiple physical and psychological comorbidities, such as metabolic syndrome, psoriatic arthritis, and depression.3 Patients with inadequately managed psoriasis can experience substantial burden of illness, with reductions in quality of life similar to those experienced by patients with diabetes or cancer,4 and patients may experience social stigmatization, sometimes magnified by anticipation of rejection and feelings of guilt and shame.5
The ultimate goal of psoriasis management is complete clearance of skin symptoms, and the consensus definition for maintaining a patient’s current psoriasis therapy is achievement of 75% (or 50% in some cases) reduction in psoriasis area and severity index from baseline.6 Current therapeutic options include a variety of topical therapies, and both American and European guidelines recommend combination vitamin D3 analogues and topical corticosteroids, such as fixed combination calcipotriol 50 µg/g (as hydrate; Cal) plus betamethasone 0.5 mg/g (as dipropionate; BD), as first-line topical therapy for patients with mild to moderate psoriasis.6-8 Fixed combination Cal/BD in both ointment and gel (topical suspension) formulations has been studied extensively in the first-line topical setting and provides fast and effective control that is more efficacious than its component monotherapies.9-11 A new alcohol-free aerosol foam formulation of fixed combination Cal/BD has been developed for the topical treatment of psoriasis vulgaris. New methods of psoriasis drug delivery are the subject of ongoing research,12 and the fixed combination Cal/BD aerosol foam formulation has been developed to enhance delivery of the active components onto the skin and potentially provide a more cosmetically acceptable alternative for patients than currently available ointment and gel formulations.
In rare cases, the use of topical corticosteroids and vitamin D3 analogues has been associated with reversible suppression of the hypothalamic-pituitary-adrenal (HPA) axis13 and calcium changes (hypercalcaemia/hypercalciuria), respectively.14 Studies have shown that foam formulations provide enhanced penetration15,16 and that Cal/BD foam provides significant improvement in antipsoriatic effects compared with earlier formulations16,17; consequently, evaluation for potential systemic adverse events (AEs) is an important element in the clinical investigation of this new product. The objective of this maximal-use systemic-exposure (MUSE) trial was to assess the systemic safety of Cal/BD aerosol foam via evaluation of HPA axis function and calcium homeostasis in maximum-use conditions.
Methods
Patients
Adult patients (aged ≥18 years) with extensive psoriasis vulgaris of at least moderate disease severity according to the Physician’s Global Assessment of Disease Severity (PGA) on the trunk, limbs, and scalp were enrolled. Extensive disease was defined as total involvement of between 15% and 30% of the body surface area (BSA), including a minimum of 30% scalp involvement. Psoriatic lesions of the face, genitals, and skin folds were not included in the BSA assessment. Patients were required to exhibit normal HPA axis function, including serum cortisol levels greater than 138 nmol/L before adrenocorticotropic hormone (ACTH) challenge and serum cortisol levels greater than 497 nmol/L 30 minutes after an ACTH challenge test during screening, as well as albumin-corrected serum calcium levels below the upper limit of normal. Patients had psoriasis for a minimum of 6 months and were amenable to topical treatment with a maximum of 120 g of trial medication per week.
Patients with a known or suspected condition affecting cortisol levels or HPA axis integrity (depression and endocrine disorders such as Cushing’s disease, Addison’s disease, or diabetes mellitus) or disorders of calcium metabolism associated with hypercalcaemia could not enroll. Washout periods for treatments known to affect cortisol levels or HPA axis integrity, calcium metabolism, or psoriasis (excluding emollients) were routine and ranged between 2 and 8 weeks prior to entry; some biologics were forbidden up to 16 weeks prior to treatment. Please see the supplementary material for more details (available online at http://cms.sagepub.com).
Study Design
This was a multicentre, open-label, single-arm, 4-week trial enrolling patients with psoriasis from 8 centres in Canada. Patients applied Cal/BD aerosol foam once daily to all lesions on the trunk, limbs, and scalp (excluding skin folds, face, and genitals). Training on how to apply treatment and first treatment application occurred at treatment baseline (day 0). Baseline for laboratory assessments was screening visit 2, which was held between 3 and 7 days before the first treatment application (for more information, see the study design figure in the supplementary material).
Patients returned for assessment at weeks 2 and 4 and, if required, for a safety follow-up visit either 2 or 4 weeks after study day 28. The institutional review boards or independent ethics committees of all investigational sites approved the protocol. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice. The clinicaltrials.gov study identifier is NCT01600222.
Study Objectives
The study objective was to evaluate the effect of once-daily use of Cal/BD aerosol foam on the HPA axis and calcium homeostasis in patients with extensive psoriasis vulgaris. Other objectives included assessment of safety by AEs and standard clinical laboratory tests, pharmacokinetics (PK), and efficacy.
The primary endpoints were patients with serum cortisol levels 497 nmol/L or less at 30 minutes after the ACTH challenge test at week 4 as well as changes from baseline to week 4 in albumin-corrected serum calcium, 24-hour urinary calcium excretion, and ratio of urinary calcium to creatinine. Secondary endpoints included serum cortisol concentration of 497 nmol/L or less at 30 and 60 minutes after the ACTH challenge test at week 4 and PK assessment. Additional secondary endpoint assessments were changes in levels of serum phosphate, serum alkaline phosphatase (ALP), plasma parathyroid hormone (PTH), 24-hour urinary phosphate excretion, and urinary phosphate-creatinine ratio from baseline to week 4.
Assessments
An ACTH challenge test using a standard intravenous injection of cosyntropin 250 mg was conducted at baseline and week 4 to evaluate HPA axis function. Serum cortisol levels were evaluated before stimulation and 30 and 60 minutes after stimulation. Potential adrenal suppression was indicated if serum cortisol levels were 497 nmol/L or less at 30 minutes after the injection. Cortisol assay details are available in the supplementary material. Evaluation of albumin-corrected serum calcium, 24-hour urinary excretion of calcium, and urinary calcium-creatinine ratio, as well as other calcium-related parameters (serum phosphate, serum ALP, plasma PTH, 24-hour urinary phosphate, urinary phosphate-creatinine ratio) was performed at baseline and week 4.
Severity of psoriasis was evaluated according to the 5-point PGA scale (clear, almost clear, mild, moderate, severe) at baseline, week 2, and week 4. Treatment success was defined as achievement of clear or almost clear on PGA. AEs were recorded throughout the study and coded in accordance with the current version of the Medical Dictionary for Regulatory Activities.
Predose blood samples drawn at baseline, week 2, and week 4 were used to assess PK. At week 4, blood samples were also collected 1, 2, 3, 5, and 7 hours after Cal/BD aerosol foam application. Plasma concentrations of BD and Cal and their main metabolites betamethasone 17-propionate and MC1080 were analyzed using a sensitive, specific, and validated bioanalytical method involving liquid chromatography with tandem mass spectrometry (data on file at LEO Pharma). The bioanalysis was performed at ICON Development Solutions (Whitesboro, New York, USA). The lower limit of quantification (LLOQ) was 30 pg/mL for BD and betamethasone 17-propionate, 50 pg/mL for Cal, and 20 pg/mL for MC1080.
Statistical Analysis
A sample size of 30 patients providing data for the ACTH challenge test at the end of treatment was regarded as adequate for an HPA axis study. Statistical analyses were descriptive. In the analysis of the efficacy endpoint, the last-observation-carried-forward method was used to replace any missing values at the end-of-treatment visit. For all other endpoints, an observed-cases approach was adopted.
Results
Patient Characteristics
Thirty-seven patients (mean age 48 years; 46% female) were enrolled and received Cal/BD aerosol foam; 35 patients completed the study per protocol. Two patients were withdrawn from treatment after 14 days; 1 patient had serum cortisol levels 497 nmol/L or less at the 30-minute test following the ACTH challenge test at baseline, and 1 patient took prohibited concomitant medication (escitalopram). At baseline, 24% of patients had severe disease according to PGA, and mean BSA involvement was 21% (Table 1) (definitions of PGA categories can be found in the table in the supplementary material). A mean of 62 g/wk (range, 13.5-113 g/wk) Cal/BD aerosol foam was used during the study.
Table 1.
Patient Demographics and Disease Characteristics at Baseline.
| Description | Statistic |
|---|---|
| Randomized, n (%) | 37 (100) |
| Female | 17 (46) |
| Male | 20 (54) |
| Mean age (SD), y | 48 (14) |
| Race, n (%) | |
| White | 30 (81) |
| Black or African American | 2 (5) |
| Other, including Asian, American Indian, or Alaska Native | 5 (14) |
| Ethnicity, n (%) | |
| Not Hispanic or Latino | 36 (97) |
| Hispanic or Latino | 1 (3) |
| Fitzpatrick skin type,a n (%) | |
| I | 4 (11) |
| II | 10 (27) |
| III | 16 (43) |
| IV | 4 (11) |
| V | 1 (3) |
| VI | 2 (5) |
| Mean duration of psoriasis (SD), y | 16 (12) |
| PGA at baseline, n (%) | |
| Moderate | 28 (76) |
| Severe | 9 (24) |
| Psoriasis involvement, mean (SD), % body surface area | |
| Trunk and limbs | 18 (5) |
| Scalp | 3 (2) |
| Total | 21 (5) |
| Psoriasis involvement on scalp, mean (SD), % scalp | 50 (23) |
Skin type classification: type I (white: always burns easily; never tans); type II (white: always burns easily; tans minimally); type III (white: burns moderately; tans gradually [light brown]); type IV (white: burns minimally; always tans well [moderate brown]); type V (brown: rarely burns; tans profusely [dark brown]); type VI (black: never burns; deeply pigmented). Definition of Physician’s Global Assessment of Disease Severity (PGA) categories can be found in the supplementary material.
Safety
HPA Axis and Calcium Homeostasis
All of the 35 patients who reached week 4 exhibited a normal response to ACTH stimulation (serum cortisol >497 nmol/L 30 minutes after ACTH stimulation) (Figure 1). Normal responses were also seen 60 minutes after ACTH stimulation.
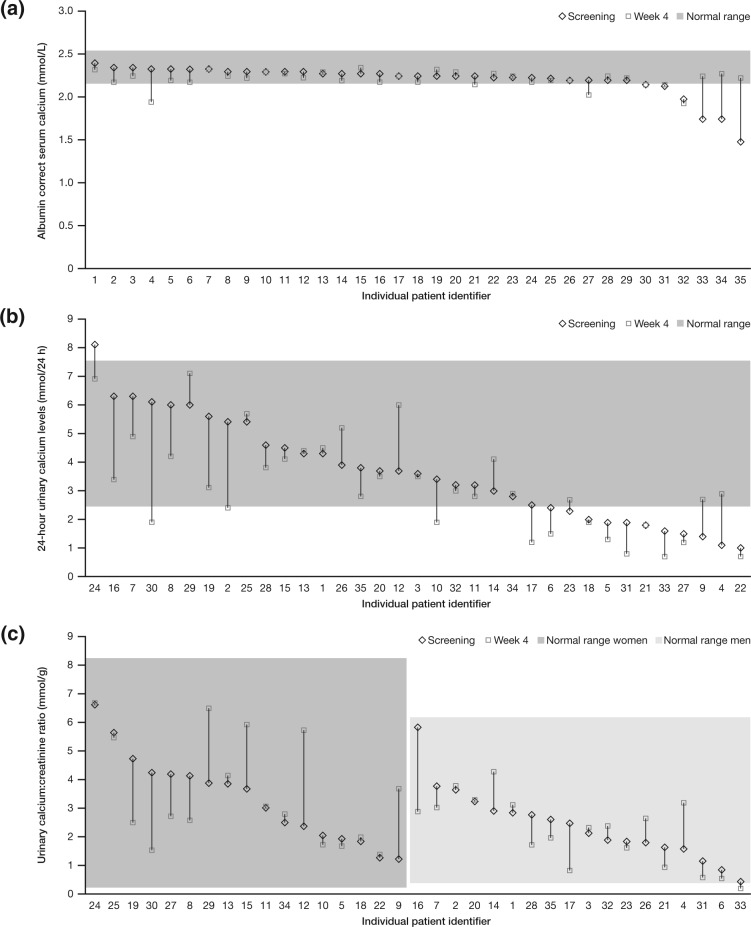
Figure 1.
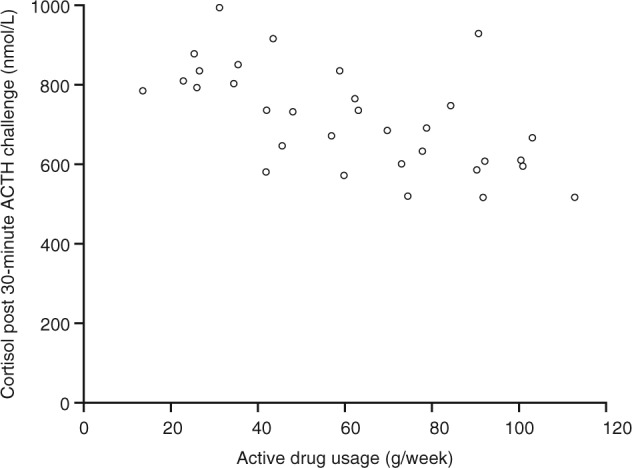
Serum cortisol levels 30 minutes after adrenocorticotropic hormone (ACTH) challenge test at week 4 versus average weekly drug use. Data are from patients who completed the study per protocol and with drug usage data (n = 32). The cortisol assay used was the Siemens ADVIA Centaur XP.
Median albumin-corrected serum calcium levels were within the normal range (2.15-2.55 mmol/L) at baseline and week 4 (2.23 mmol/L; interquartile range [IQR], 2.18-2.28), with a median decrease of −0.02 mmol/L. Median 24-hour urinary calcium levels were within the normal range (2.5-7.5 mmol/24 h) at baseline and week 4 (2.9 mmol/24 h; IQR 1.9-4.2), with a median decrease of −0.3 mmol/24 h. The median urinary calcium-creatinine ratio was within the reference range (0.225-8.2 mmol/g for women and 0.3-6.1 mmol/g for men) at baseline and at week 4 (2.65 mmol/g; IQR, 1.68-3.68), with a median increase of 0.05 mmol/g.
No patients had elevations above the normal range in any calcium homeostasis parameter at week 4. Four of 6 patients with low albumin-corrected serum calcium levels at baseline had levels shifted into the normal range by week 4 (Figure 2a; some patients had levels shifting in the opposite direction). Shifts in 24-hour urinary calcium were observed in both directions (Figure 2b), and no increases above the normal range were seen in the calcium-creatinine ratio (Figure 2c).
Figure 2.
Individual patient (a) albumin-corrected serum calcium values, (b) 24-hour urinary calcium levels, and (c) urinary calcium-creatinine ratios at screening visit 2 (ie, baseline) and at week 4. Data not shown for patients who discontinued after 2 weeks of treatment.
The secondary calcium homeostasis endpoints were the changes from baseline to week 4 in serum phosphate, serum ALP, plasma PTH, 24-hour urinary phosphate excretion, and urinary phosphate-creatinine ratio. The median levels for each of the secondary calcium homeostasis parameters were within the reference ranges at both baseline and week 4, and the median changes from baseline to week 4 were small and not considered clinically significant. A small number of patients had shifts from the normal to high category for 24-hour urinary phosphate excretion (1 patient), urinary phosphate-creatinine ratio (1 patient), serum ALP (1 patient), and plasma PTH (4 patients). One patient had a shift from a normal 24-hour urinary phosphate excretion value at baseline to a low value at week 4. None of these shifts were considered clinically relevant.
AEs and Standard Safety Laboratory Tests
All patients were analyzed for safety parameters (safety analysis set included all 37 patients). Four patients (11%) reported 6 AEs of mostly mild or moderate intensity, and no specific AE was experienced by more than 1 patient (Table 2). One of the events, severe erythema, which occurred within 4 days of treatment, was considered related to treatment. The other 5 AEs were considered not related to treatment. There were no clinically significant changes in haematologic, biochemical, or urinalysis parameters.
Table 2.
Adverse Events Experienced During the Triala.
| Adverse Event | n (%) |
|---|---|
| Fungal infection | 1 (3) |
| Arthralgia | 1 (3) |
| Headache | 1 (3) |
| Oropharyngeal pain | 1 (3) |
| Upper-airway cough syndrome | 1 (3) |
| Erythema | 1 (3) |
Medical Dictionary for Regulatory Activities, version 15.1, was used for coding of adverse events.
Pharmacokinetics
All 35 patients who completed the study per protocol were included in the PK analysis. Measurable plasma concentrations of Cal, BD, and the Cal metabolite MC1080 were available at some time points in 1, 5, and 3 patients, respectively. Twenty-seven patients had measureable concentrations of the BD metabolite betamethasone 17-propionate, but only values for maximal plasma concentration and area under the plasma concentration-time curve from zero to last quantifiable concentration (148 pg/mL and 68.4 h·pg/mL, respectively) could be determined.
Efficacy
The vast majority of patients showed improvement in their disease severity compared with baseline, and 49% achieved treatment success (clear or almost clear disease) according to the PGA at week 4. Mild disease was achieved by 37% of patients, and the remaining 14% of patients had moderate disease at week 4. By week 2, 11% of patients had already achieved treatment success and 70% had mild disease. The percentage of patients in each PGA category is summarized in Figure 3.
Figure 3.
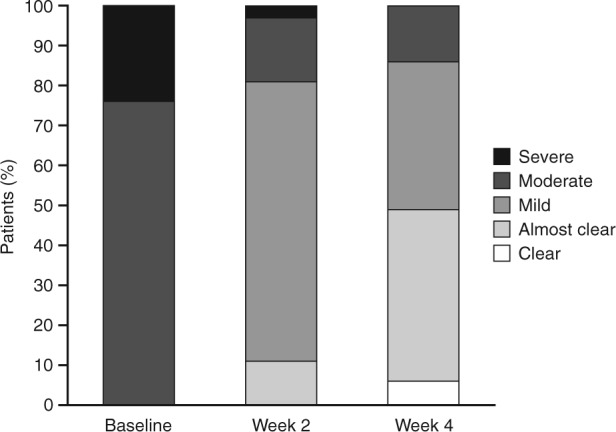
Percentage of patients in each category of the Physician’s Global Assessment of Disease Severity at baseline, week 2, and week 4.
Discussion
This MUSE trial found that maximal-dose systemic exposure to Cal/BD aerosol foam resulted in no clinically relevant impact on the HPA axis and calcium homeostasis. The favourable results found in this study are similar to those seen in previous MUSE and other studies of Cal/BD provided as ointment and gel (topical suspension).11,18-20
MUSE studies are designed to investigate the safety impact of drugs when applied at the upper limit of topical exposure.21 As such, inclusion of patients with extensive disease (15%-30% of BSA) was required to ensure that the upper range of Cal/BD dosing when applied as the aerosol foam would be evaluated in a clinical setting. A mean amount of 62 g/wk Cal/BD aerosol foam was used during the study, which is higher than the 52 g seen in a previous MUSE study of Cal/BD gel19 and the 49 to 59 g average range seen in a 4-week MUSE study of Cal/BD ointment.20 As this trial enrolled patients with extensive disease, it was considered unethical to include a placebo arm, so all patients were exposed to active treatment. Assessment bias resulting from the absence of a placebo group is considered limited, since most of the endpoints were objective in nature (laboratory data).
All patients who completed the current trial exhibited a normal response to ACTH stimulation, and no patients had levels above the normal reference range in albumin-corrected serum calcium, 24-hour urinary calcium, or urinary calcium-creatinine ratio at week 4. Changes in all median and individual calcium homeostasis values from baseline to week 4 were minor and not considered clinically relevant. The findings with Cal/BD aerosol foam in the current study seem encouraging because, despite enhanced penetration and the improved antipsoriatic effect with the aerosol foam formulation,15,16 the systemic safety profile is similar to that seen with the gel formulation, with no or minimal effects on the HPA axis and calcium homeostasis.19 Cal/BD aerosol foam also demonstrated a good tolerability profile, similar to that seen with the previous fixed combination products.11,18,22
Adrenal suppression has previously been observed in the setting of high-dose Cal/BD in a few patients.11 A MUSE study of the gel formulation identified 2 patients showing signs of adrenal suppression, although it was anticipated that only a few patients would experience this effect in the real-world setting.19 Another MUSE study evaluating the Cal/BD ointment formulation found that no patients experienced adrenal suppression when Cal/BD ointment was applied once daily.20 A long-term non-MUSE study found no evidence of adrenal suppression related to treatment with Cal/BD ointment when assessed in a subgroup of 19 patients treated once daily as needed for 52 weeks.20 Thus, the systemic safety profile of gel and ointment Cal/BD products indicates minimal risk of systemic adverse reactions within the recommended doses, and there is no indication of any greater effects with the Cal/BD aerosol foam formulation.
Changes in calcium homeostasis have been infrequently reported in Cal/BD studies. As reported by McCormack,11 no clinically relevant changes in albumin-corrected serum calcium levels were observed in any of the Cal/BD studies reviewed. Additionally, no clinically relevant mean changes in parameters of calcium homeostasis (serum calcium, 24-hour urinary calcium, and calcium-creatinine ratio) were observed in the MUSE Cal/BD gel study.19
Efficacy and PK parameters were evaluated as secondary endpoints. PK analysis was possible in only a subset of patients as a result of study drug and metabolite plasma concentrations below the LLOQ. In the MUSE study of Cal/BD gel, the same analytes were evaluated (Cal, BD, and major metabolites MC1080 and betamethasone 17-propionate) and no PK parameters could be calculated because too few patients had plasma concentrations above the LLOQ.19
In the current study, the vast majority of patients experienced disease improvement and almost half of patients achieved treatment success (clear or almost clear disease) with Cal/BD aerosol foam by week 4. This is an impressive finding for this group of patients with extensive disease (average BSA of 21%, with 24% of patients experiencing severe disease at baseline). Additionally, this improvement was achieved over a short time period—4 weeks. Despite the large surface area treated using the enhanced foam aerosol vehicle, this study showed no evidence of unwanted systemic effects, with a safety and tolerability profile similar to that previously shown for Cal/BD in ointment and gel vehicles.
Further phase II studies powered to evaluate whether Cal/BD aerosol foam provides an efficacy benefit to patients have shown Cal/BD aerosol foam to be significantly more effective than its monocomponents Cal aerosol foam and BD aerosol foam in providing physician-assessed treatment success23 and have shown that Cal/BD aerosol foam provides greater efficacy and similar tolerability compared with Cal/BD ointment.24 Additional studies have provided evidence supporting enhanced drug delivery with Cal/BD aerosol foam.16,17 The Cal/BD aerosol foam formulation may provide patients with an important new topical treatment option, as convenient and cosmetically elegant formulations, particularly foam and solution vehicles, have performed well in patient preference tests.25
Conclusions
In patients with extensive psoriasis vulgaris treated once daily for 4 weeks, Cal/BD aerosol foam exhibited no clinically relevant impact on the HPA axis or calcium homeostasis. Forty-nine percent of patients achieved treatment success (clear or almost clear status) when evaluated for efficacy. Cal/BD foam also demonstrated a favourable tolerability profile.
Supplementary Material
Acknowledgments
This study was sponsored by LEO Pharma. We would like to thank the study investigators: V. Taraska, M. Raman, J. Arseneau, R. Tuppal, K. Papp, L. Albrecht, A. Gupta, and M. Gilbert. We also thank Mette Kielsholm Fife from LEO Pharma for her role as the international clinical trial manager.
Footnotes
Authors’ Note: Presented at Fall Clinical Dermatology Conference, October 16-19, 2014, Las Vegas, Nevada; and Skin Disease Education Foundation 15th Annual Las Vegas Dermatology Seminar, October 30-November 1, 2014, Las Vegas, Nevada.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Taraska has been a speaker/investigator for LEO Pharma, Basilea, Amgen, Galderma, AbbVie, Allergan, Cipher, and Valeant. Dr Tuppal has been a speaker/investigator for Amgen, Pfizer, LEO Pharma, Galderma, Valeant, Novartis, and Schering (MSD). Dr Olesen and Dr Bang Pedersen are employees of LEO Pharma. Dr Papp has been a consultant for Janssen, Johnson & Johnson, Kirin, Kyowa, LEO Pharma, Lypanosys, Merck, Mitsubishi Pharma, Novartis, Pan Genetics, Pfizer, Roche, Merck Serono, Takeda, UCB, and Vertex; a speakers’ bureau member for Janssen, Merck, Novartis, and Pfizer; an investigator for Janssen, Kyowa, LEO Pharma, Merck, Novartis, Pfizer, Stiefel, and Takeda; a speaker for Janssen, Kyowa, Lypanosys, Merck, Mitsubishi Pharma, Novartis, Pfizer, Merck Serono, Takeda, UCB, Vertex, and Wyeth; a steering committee member for Janssen, Kirin, Kyowa, Merck, Novartis, and Pfizer; and an advisory board member for Janssen, Merck, Novartis, Pfizer, and UCB.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by LEO Pharma. Writing assistance was provided by Helene Wellington from Mudskipper Business Ltd, funded by LEO Pharma.
References
- 1. Parisi R, Symmons DP, Griffiths CE, et al. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. [DOI] [PubMed] [Google Scholar]
- 2. World Health Assembly. Psoriasis. Agenda item 13.5. WHA67.9. 2014. http://appswhoint/gb/ebwha/pdf_files/WHA67/A67_R9-en.pdf. Accessed February 26, 2015.
- 3. Kimball AB, Gieler U, Linder D, et al. Psoriasis: is the impairment to a patient’s life cumulative? J Eur Acad Dermatol Venereol. 2010;24:989-1004. [DOI] [PubMed] [Google Scholar]
- 4. Raho G, Koleva DM, Garattini L, et al. The burden of moderate to severe psoriasis: an overview. Pharmacoeconomics. 2012;30:1005-1013. [DOI] [PubMed] [Google Scholar]
- 5. Hrehorow E, Salomon J, Matusiak L, et al. Patients with psoriasis feel stigmatized. Acta Derm Venereol. 2012;92:67-72. [DOI] [PubMed] [Google Scholar]
- 6. Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Girolomoni G, Vena GA, Ayala F, et al. Consensus on the use of the fixed combination calcipotriol/betamethasone dipropionate in the treatment of plaque psoriasis. G Ital Dermatol Venereol. 2012;147:609-624. [PubMed] [Google Scholar]
- 8. Papp K, Gulliver W, Lynde C, et al. Canadian guidelines for the management of plaque psoriasis: overview. J Cutan Med Surg. 2011;15:210-219. [DOI] [PubMed] [Google Scholar]
- 9. Hendriks AG, Keijsers RR, de Jong EM, et al. Efficacy and safety of combinations of first-line topical treatments in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;27:931-951. [DOI] [PubMed] [Google Scholar]
- 10. Fleming C, Ganslandt C, Guenther L, et al. Calcipotriol plus betamethasone dipropionate gel compared with its active components in the same vehicle and the vehicle alone in the treatment of psoriasis vulgaris: a randomised, parallel group, double-blind, exploratory study. Eur J Dermatol. 2010;20:465-471. [DOI] [PubMed] [Google Scholar]
- 11. McCormack PL. Calcipotriol/betamethasone dipropionate: a review of its use in the treatment of psoriasis vulgaris of the trunk, limbs and scalp. Drugs. 2011;71:709-730. [DOI] [PubMed] [Google Scholar]
- 12. Katare OP, Raza K, Singh B, et al. Novel drug delivery systems in topical treatment of psoriasis: rigors and vigors. Indian J Dermatol Venereol Leprol. 2010;76:612-621. [DOI] [PubMed] [Google Scholar]
- 13. Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51. [DOI] [PubMed] [Google Scholar]
- 14. Kragballe K, Iversen L. Calcipotriol: a new topical antipsoriatic. Dermatol Clin 1993;11:137-141. [PubMed] [Google Scholar]
- 15. Huang X, Tanojo H, Lenn J, et al. A novel foam vehicle for delivery of topical corticosteroids. J Am Acad Dermatol. 2005;53:S26-S38. [DOI] [PubMed] [Google Scholar]
- 16. Hollesen Basse L, Olesen M, Lacour JP, et al. Enhanced in vitro skin penetration and antipsoriatic effect of fixed combination calcipotriol plus betamethasone dipropionate in an innovative foam vehicle. J Invest Dermatol. 2014;134:S33. Abstract 192. [Google Scholar]
- 17. Queille-Roussel C, Olesen M, Villumsen J, et al. Efficacy of an innovative aerosol foam formulation of fixed combination calcipotriol plus betamethasone dipropionate in patients with psoriasis vulgaris. Clin Drug Investig. 2015;35:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kragballe K, Austad J, Barnes L, et al. A 52-week randomized safety study of a calcipotriol/betamethasone dipropionate two-compound product (Dovobet®/Daivobet®/Taclonex®) in the treatment of psoriasis vulgaris. Br J Dermatol. 2006;154:1155-1160. [DOI] [PubMed] [Google Scholar]
- 19. Silver S, Tuppal R, Gupta AK, et al. Effect of calcipotriene plus betamethasone dipropionate topical suspension on the hypothalamic-pituitary-adrenal axis and calcium homeostasis in subjects with extensive psoriasis vulgaris: an open, non-controlled, 8-week trial. J Drugs Dermatol. 2013;12:882-887. [PubMed] [Google Scholar]
- 20. Fleming C, Ganslandt C, Leese GP. Short- and long-term safety assessment of a two-compound ointment containing calcipotriene/betamethasone dipropionate (Taclonex®/Daivobet®/Dovobet® ointment): hypothalamic-pituitary-adrenal axis function in patients with psoriasis vulgaris. J Drugs Dermatol. 2010;9:969-974. [PubMed] [Google Scholar]
- 21. FDA. FDA guidance to industry. Acne vulgaris: developing drugs for treatment. 2005. http://wwwfdagov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM071292.pdf. Accessed June 5, 2014.
- 22. Douglas WS, Poulin Y, Decroix J, et al. A new calcipotriol/betamethasone formulation with rapid onset of action was superior to monotherapy with betamethasone dipropionate or calcipotriol in psoriasis vulgaris. Acta Derm Venereol. 2002;82:131-135. [DOI] [PubMed] [Google Scholar]
- 23. Lebwohl M, Tyring S, Bukhalo M, et al. , et al. A novel aerosol foam formulation of calcipotriene (Cal) 0.005% plus betamethasone dipropionate (BD) 0.064% is more efficacious than Cal and BD foam alone in treating psoriasis vulgaris: a randomized, double-blind, multicenter, three-arm, phase II study. Paper presented at: 73rd Annual Meeting of the American Academy of Dermatology (AAD); March 20-24, 2015; San Francisco, CA. Abstract 1670. [Google Scholar]
- 24. Koo J, Tyring S, Werschler WP. Superior efficacy of the fixed combination calcipotriene plus betamethasone dipropionate in an innovative aerosol foam versus ointment, in patients with psoriasis vulgaris. Abstract presented at: Skin Disease Education Foundation’s 39th Annual Hawaii Dermatology Seminar; March 1-6, 2015; Koloa, HI. [Google Scholar]
- 25. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.