Summary
Nuclear Dbf2-related (NDR) kinases play a central role in limiting growth in most animals. Signals that promote growth do so in part by suppressing the activation of NDR kinases by STE20/Hippo kinases. Here, we identify another mechanism for down-regulating NDR kinase activity. Specifically, we show that activity of the Drosophila NDR kinase Warts in the developing wing depends on its transition from an inactive “closed” to a potentially active, “open” conformation mediated by Mats, a conserved Mps1-binder (Mob) protein. Further, we show that signaling interactions between the protocadherins Fat and Dachsous, organized by the morphogens Wingless and Decapentaplegic, suppress Warts by acting via the atypical myosin Dachs to inhibit or reverse this transition. The regulation of Warts conformation by Mats, Fat/Dachsous signaling and Dachs appears independent of Warts phosphorylation by Hippo kinase, establishing a precedent for the control of NDR kinases, and hence growth, by distinct allosteric and phosphorylation mechanisms.
Graphical abstract
Introduction
Organ growth is a poorly understood process in which each body part increases in mass and cell number until it reaches a tightly controlled final size. Morphogens, such as secreted molecules of the Wnt, BMP and Hh superfamilies, organize growth: little or no growth occurs in their absence, and their ectopic expression results in adventitious growth (Lawrence and Struhl, 1996; Tabata and Takei, 2004). Conversely, Nuclear Dbf2-related (NDR) kinases, such as Drosophila Warts (Wts) and mammalian Large Tumor Suppressor proteins (Lats1/2), play an opposing role in constraining growth [(Justice et al., 1995; Xu et al., 1995); reviewed in (Pan, 2010; Staley and Irvine, 2012; Enderle and McNeill, 2013; Hergovich, 2013)]. Indeed, functional studies in Drosophila suggest that morphogens promote growth at least in part by suppressing Wts kinase activity (Rogulja et al., 2008; Zecca and Struhl, 2010).
The conserved NDR kinase signal transduction module contains two additional components, Ste20/Hippo (Hpo) kinases (Chan et al., 2005; Stegert et al., 2005), and Mps1-one Binder (Mob) proteins (Bichsel et al., 2004; He et al., 2005). Hpo kinases phosphorylate NDR kinases and are thought to trigger catalytic activity by restructuring the kinase domain (Yang et al., 2002b). The role of Mob proteins is less well understood. They have been proposed to facilitate the association of NDR kinases with other activators or their release from an auto-inhibited state (Bichsel et al., 2004; Lai et al., 2005).
A central challenge in animal development has been to understand how morphogens control organ growth by regulating NDR kinase activity. NDR kinases are governed by diverse upstream modulators that exert their influence by regulating Hpo kinase activity (Pan, 2010; Halder et al., 2012; Staley and Irvine, 2012; Enderle and McNeill, 2013; Hergovich, 2013), or the capacity of Hpo kinase to gain access to NDR kinases (Yin et al., 2013). However, it is not known if or how morphogens operate via any of these modulators to regulate growth.
In contrast, studies in Drosophila have linked morphogens to the control of growth via regulation of the NDR kinase Wts by the protocadherins Fat (Ft) and Dachsous (Ds) (Bennett and Harvey, 2006; Cho et al., 2006; Willecke et al., 2006; Rogulja et al., 2008; Willecke et al., 2008; Zecca and Struhl, 2010). Normally, morphogen signaling generates opposing differentials of Ft and Ds signaling in developing tissues (Clark et al., 1995; Vilano and Katz, 1995; Casal et al., 2002; Yang et al., 2002a). These differentials are transduced by the atypical myosin Dachs, which promotes growth by inhibiting Wts (Cho et al., 2006; Mao et al., 2006; Rogulja et al., 2008; Zecca and Struhl, 2010).
Here, we have dissected how morphogens regulate NDR kinases by asking if and how the activity of Drosophila Wts depends on its allosteric state. To do so, we engineered a functional “bio-sensor” form of Wts that uses Fluorescence Resonance Energy Transfer (FRET) to report on its conformation when expressed at physiological levels during normal wing development.
Using our Wts-FRET sensor, we have obtained evidence for two distinct states of Wts, a “closed”, inactive form, and an “open”, potentially active form. Further, we have determined that Mats, a Drosophila Mob protein, converts Wts from the “closed” to the “open” state, and that Ft/Ds signaling operates via Dachs to suppress Wts activity by inhibiting or reversing the transition mediated by Mats. Conversely, we find that Hpo phosphorylation, although required for Wts kinase activity, has no apparent effect on the conformation of Wts as monitored by the sensor. Thus, we have identified a mechanism for regulating NDR kinases that depends on allosteric change effected by Mob protein activity rather than phosphorylation catalyzed by Hpo kinases. Further, this mechanism links morphogen to the control of growth via the action of Ft/Ds signaling and Dachs on the conformation of Wts.
Results
The Quenching Ratio (Q) as a measure of Wts-FRET sensor conformation in vivo
To determine if activation of Drosophila Wts correlates with a conformational change, we engineered a fully functional form of Wts tagged with monomeric Citrine (mCitrine) at the amino- terminus, and monomeric Teal (mTFP1) at the carboxy- terminus (mCitrine-Wts-mTFP1, henceforth CWT; Fig. 1A). The two fluorescent proteins can function as a fluorescence resonance energy transfer (FRET) pair (mTFP1, donor; mCitrine, acceptor) (Ai et al., 2008), providing the potential to detect changes in the proximity or relative orientation of the two Wts termini. We expressed CWT in vivo under the control of either the Tubulinα1 (Tubα1) or ribosomal protein 49 gene (rp49) promoters to generate uniform levels similar to that of endogenous Wts (Experimental Procedures). These transgenes fully rescue the development of wts null animals to phenotypically normal, fertile adults, and unless otherwise stated, all experiments aside from those involving RNAi knockdown or mutated, non-functional forms of the CWT sensor were performed using such “CWT rescued”, wts null animals. At the subcellular level, CWT behaves like native Wts (Rauskolb et al., 2014), accumulating predominantly in association with the apical cell membrane (e.g., Fig. 1D, CWT image), but also present at a lower level in the cytosol.
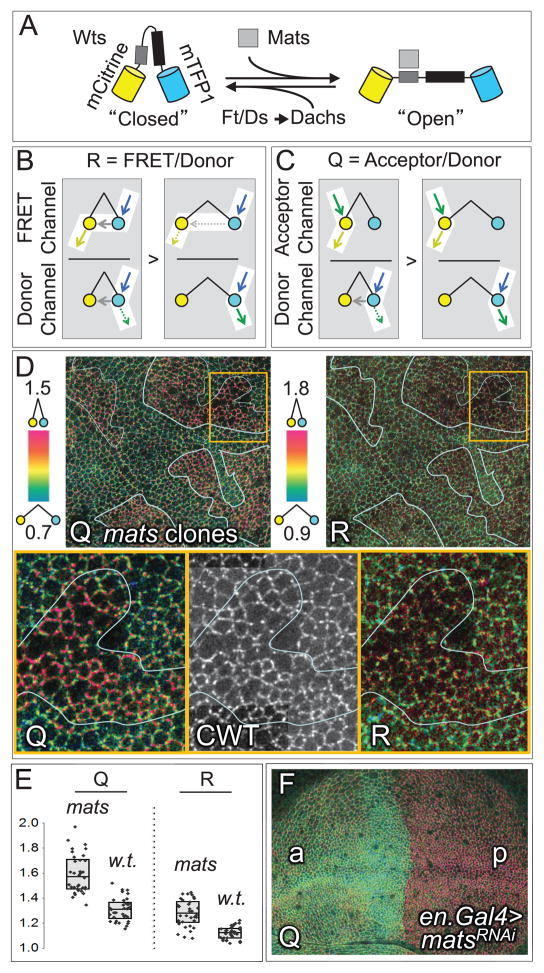
Figure 1. Conformational state analysis of Wts using the CWT FRET sensor.
A) The CWT Wts-FRET sensor is composed of full length Wts fused to mCitrine (yellow) and mTFP1 (turquoise) at its amino- and carboxy-termini; Mats and the Mats binding domain are shown as grey boxes, and the Wts kinase domain as a black box. Also shown is the putative “closed” to “open” transition promoted by Mats and inhibited or reversed by Ft/Ds signaling via Dachs. We depict these states as “closed” and “open” for convenience, consistent with prior evidence that the amino- and carboxy- termini of the NDR kinase Lats1 can interact (Tao et al., 1999). However, any conformational change that alters the proximity or orientation of the Wts amino- and carboxy- termini may alter energy transfer monitored by the CWT Wts-FRET sensor.
B, C) The excitation inputs and emission outputs of the donor, acceptor, and FRET channels are diagrammed for both the “closed” and the “open” CWT configurations. Excitation photons are depicted by blue (mTFP1) and green (mCitrine) arrows; emission photons, by green (mTFP1) and yellow (mCitrine) arrows; resonant energy transfer, by gray arrows. Dashed arrows indicate lower emissions or energy transfers. B) R is the ratio of emissions detected in the FRET channel relative to the donor channel and increases as a function of proximity-dependent energy transfer, visualized in subsequent figures by a green-to-red color scale. C) Q is the ratio of acceptor channel to donor channel emissions and, like R, rises as a result of increased energy transfer.
Evidence for distinct “open” and “closed” states of Wts in the presence or absence of Mats.
D) Intensity modulated displays of Q and R ratios for the CWT sensor on the apical surface of a wing primordium containing large clones of matse03077 cells (top panels), color coded as indicated to the left of each image; here, and in subsequent figures, clones were marked by expression of myristoylated RFP (mRFP, as in Figs. S3–S5, S7, S8). A portion of a single clone, boxed in orange, is shown at higher magnification (bottom panels), together with an image of the acceptor channel, indicating total protein (CWT).
Cells within mats mutant clones appear redder than surrounding wildtype cells, indicating higher, proximity-dependent energy transfer in the absence of Mats, consistent with increased occupancy in the “closed” CWT state (further supporting data presented in Fig. S1). This difference is more pronounced for Q than for R.
E) R and Q measurements derived from 30 cellular interfaces located either within matse03077or neighboring wildtype tissue, as indicated. The boxes display the median (horizontal line through the box) as well as the interquartile range (top and bottom edges); individual measurements are shown as black diamonds.
F) Q ratio image for a wing primordium in which mats expression was knocked down in the posterior compartment by matsRNAi (under en.Gal4/UAS control). matsRNAi posterior cells (p) appear predominantly red in contrast to wildtype anterior cells (a), which appear green.
When excited at their respective optimal wavelengths, mTFP1 and mCitrine produce distinct emission profiles, which we detect in separate donor (mTFP1) and acceptor (mCitrine) “channels”, as defined in Figures 1B and 1C. When the two fluorophores are positioned favorably for energy transfer (as in the “closed” CWT conformation, Fig. 1A), excitation of mTFP1 induces emissions from mCitrine, which we detect in the FRET channel, at the expense of emissions from mTFP1, which we would otherwise detect in the donor channel (Fig. 1B). Importantly, emissions generated by direct excitation of mCitrine, which we detect in the acceptor channel (Fig. 1C), are not affected by energy transfer; hence the acceptor channel monitors the abundance of CWT protein and is impervious to changes in its conformational state.
Energy transfer using FRET is typically assessed as the FRET ratio, “R” (Campbell, 2009), which compares emissions detected in the FRET channel relative to those in the donor channel (Fig. 1B). Accordingly, R rises as a function of energy transfer because FRET channel emissions increase as donor channel emissions decline. However, assaying energy transfer using the FRET channel proved challenging when the CWT protein is expressed at physiological levels because the energy levels required to generate a detectable, bona fide signal in the FRET channel induced variable, high levels of tissue auto-fluorescence that are also detectable in this channel (typically ≥50% of the total FRET channel signal). In addition, overlaps between the excitation and emission profiles of mTFP1 and mCitrine lead to significant cross-over signal in the FRET channel that further obscures the bona fide FRET signal.
Thus, we have instead used Quenching of the donor channel signal by energy transfer to the acceptor as an indicator of conformational state (Lacowicz, 2006). Specifically, we assay the Quenching ratio (Q), computed as the ratio of emissions detected in the acceptor channel relative to emissions detected in the donor channel, so that Q, like R, rises as a function of energy transfer (Fig. 1C). Because the donor and acceptor channels detect only modest auto-fluorescent output (<20% and <5%, respectively) and are not compromised by overlaps in excitation or emission spectra, Q is not significantly affected by auto-fluorescence and excitation/emission overlaps that compromise the FRET channel signal, upon which R depends. As we document in the next section, Q is a more sensitive and reliable indicator of energy transfer under physiological conditions of CWT expression.
The Mob protein Mats is required for a conformational change in Wts
To test if Mob protein binding alters NDR kinase conformational state, we assayed CWT behavior in Drosophila wing imaginal discs containing genetically marked clones of cells that have reduced activity of Mats, the Drosophila Mob homologue required for Wts kinase activation (Lai et al., 2005). The use of clones allows the effect of reducing Mats activity in mutant cells to be compared directly with neighboring, wildtype cells across the clone border, providing an internal control for any change in Q.
Figure 1E shows a quantitative analysis comparing mutant and wildtype cells. We found that clones homozygous for matse03077, a hypomorphic allele, show a consistent, cell-autonomous increase in both Q and R. Of the two measures, Q has a better dynamic range, likely because of the factors that compromise R, as discussed above. Specifically, the difference in Q between matse03077 cells and surrounding wildtype cells (~22 %) is greater than the corresponding difference in R (~14 %; Fig. 1E).
To visualize conformational state differences in situ, we used the ratio images function of the Metamorph software to produce intensity modulated displays for both Q and R (Tsien and Harootunian, 1990) (Fig. 1D). In these images, higher ratios appear red, while lower ratios appear green/blue. For both Q and R ratio images, the apical membranes of matse03077 cells appear redder than those of the surrounding wildtype cells (Fig. 1D). As in the quantitative analysis, the differences in Q are more pronounced than those in R. A striking increase in Q was also observed when Mats activity was knocked down more effectively in the posterior compartment of the wing disc using RNAi (Fig. 1F). Given its higher dynamic range, we rely on Q, rather than R, to assess energy transfer in subsequent experiments.
Our finding that Q is higher in mats mutant tissue than in the wildtype surround indicates that the average CWT conformational state in Mats deficient cells is distinct from that in neighboring wildtype cells and supports the hypothesis that Mats is required for an activating conformational change.
For convenience, we refer to CWT within Mats-deficient cells as being in a “closed”, inactive state and to CWT in the surrounding wildtype tissue as being in an “open”, potentially active state (Fig. 1A). These designations are consistent with an increase in proximity-dependent energy transfer between the terminally located mCitrine and mTFP fluorophores in Mats-deficient cells in which Wts kinase activity is reduced (He et al., 2005; Lai et al., 2005), as well as with the capacity of the amino and carboxy termini of the NDR kinase Lats1 to bind to each other (Tao et al., 1999). However, the increase in Q observed in Mats-deficient cells might reflect some conformational change that facilitates energy transfer between mTFP1 and mCitrine other than a bona fide open to closed transition of Wts (Campbell, 2009).
Importantly, we could not detect any difference in CWT protein accumulation between matse03077 and adjacent wildtype cells, as determined by comparing emissions in the acceptor channel, which monitor the amounts of CWT protein (Fig. 1D, CWT image). Thus the increase in both Q and R in matse03077 clones, which correlates with reduced Wts activity, is not associated with a detectable change in Wts abundance. Hence, we posit that Mats controls Wts activity by altering its conformational state rather than by changing its stability.
To test this hypothesis further, we assayed Q ratios using a variant of the CWT sensor, CWTR708A, in which a single residue in the Mats interaction domain of Wts that has a conserved role in mediating NDR kinase/Mob protein interactions (Hergovich et al., 2006a; Ni et al., 2015) is mutated. When expressed under the control of the Tubα1 promoter, the CWTR708A accumulates apically like the wildtype CWT. However, in contrast to wildtype CWT, CWTR708A fails to rescue the absence of endogenous Wts, indicating that it is functionally inactive. Moreover, it shows uniformly high Q value throughout the wing disc (Fig. S1). Hence, it appears that Wts must be able to interact with Mats to transition from the “closed” to “open” state and become catalytically active.
Tissue-wide and subcellular patterns of Wts conformational state
Drosophila wing discs originate from the embryonic epidermis and proliferate extensively during larval life. The wing itself is formed by a discrete primordium within the disc, centered on the intersection of the antero-posterior (a/p) and dorso-ventral (d/v) compartment boundaries, which serve, respectively, as the sources of the morphogens Decapentaplegic (Dpp, a BMP) and Wingless (Wg, a Wnt) (Fig. 2A diagram). Dpp and Wg spread from their sources and promote wing growth in part by recruiting cells from surrounding non-wing tissue to enter the wing primordium (Zecca and Struhl, 2007). Recruitment depends on the local suppression of Wts activity at the wing periphery in response to Dpp and Wg signaling (Zecca and Struhl, 2010).
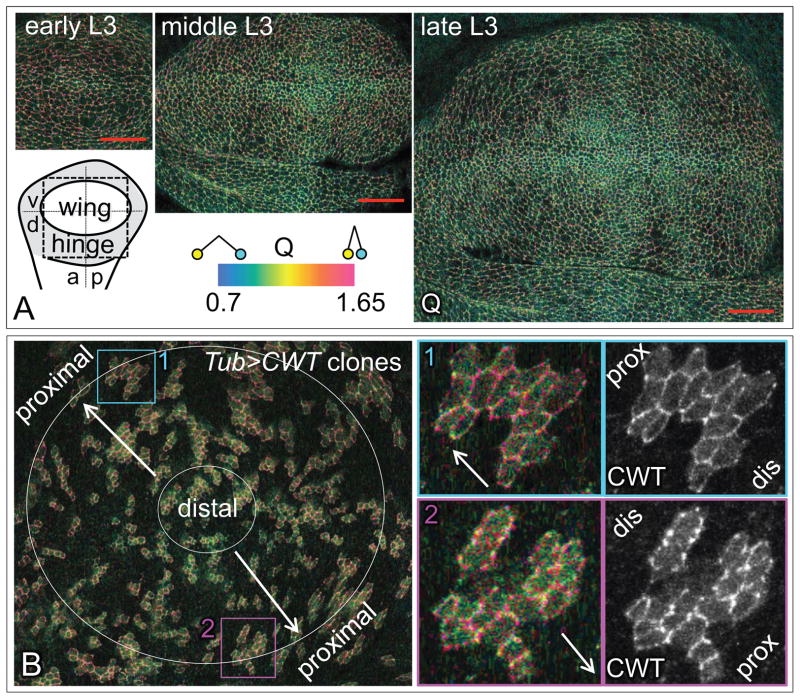
Figure 2. Tissue-wide and sub-cellular distributions of the “open” and “closed” conformational states of Wts.
A) Q ratio images for the wing and adjacent dorsal hinge primordia of discs of increasing age and size [from early, middle, and late 3rd instar larvae (L3)], as diagrammed and color coded on the lower left (a/p and d/v indicate the antero-posterior and dorso-ventral compartment boundaries; the dashed box indicates the area shown in the micrograph). Images are shown at the same magnification and scanning resolution (red scale bar = 20μm). Q is lower (greener) at the center of the wing primordium and in the vicinity of the A/P and D/V compartment borders and higher (redder) at the periphery, away from the borders.
B) Q ratio image of a wing primordium peppered with small clones of cells expressing CWT (left). Both Q and acceptor channel (CWT) images for two individual clones (turquoise and purple boxed areas 1 and 2) are shown on the right (arrows point in the distal-to-proximal direction). As in A, Q shows a general, tissue wide increase (from green to red) in the distal-to-proximal direction, but shows no obvious differential comparing the distal versus the proximal edges of small clones. See Fig. S2 for a direct, quantitative comparison of the apical accumulation of Dachs-GFP, CWT protein and Q in a single disc lightly peppered with small clones expressing Dachs-GFP or CWT.
Q ratio images show a stereotyped increase in Q from the center to the periphery of the wing primordium during the third larval instar, the major period of growth (Fig. 2A, the center appears green/blue relative to the periphery, which appears red; Q is also lower along the A/P and D/V compartment boundaries, where Dpp and Wg individually peak). Hence, there appears to be a shift in the conformation of Wts from an “open” state in the center, where the combined inputs of Dpp and Wg are maximal, towards a “closed” state at the periphery where both morphogens are limiting.
Interestingly, the increase in Q parallels a shift in the extent to which the atypical myosin Dachs accumulates asymmetrically within cells. Dachs is localized preferentially on the distal side of cells throughout the developing wing (Mao et al., 2006) but the magnitude of Dachs asymmetry shifts from being weak in the center to being strong at the periphery (Brittle et al., 2012; Merkel et al., 2014), correlating with the progressive transition in Wts from the “open” to “closed” state.
To further assess the relationship between Dachs asymmetry and Wts conformation, we analyzed Q at the subcellular level. We observed that Q is not uniform at the apical surface along a given cell-cell interface, but instead varies in punctate fashion, suggesting that CWT is enriched in either “closed” (red) or “open” (green/blue) conformational states in distinct microdomains (for example, Fig. 1D). To assess the distribution of such “closed” and “open” state enrichments relative to the distal subcellular accumulation of Dachs, we generated small, clonal populations of Tubα1>CWT expressing cells in otherwise non-CWT expressing wing discs by “Flp-out” of a Tubα1>stop>CWT transgene (Fig. 2B). At the border between cells that express and cells that do not express CWT we could thus visualize CWT on either the proximal or the distal apical membranes of individual cells. We found that although CWT enrichments are not uniformly distributed around the cell perimeter, neither the “closed” nor the “open” microdomains appear to be preferentially localized on the proximal or the distal apical membranes (Fig. 2B; Fig. S2). This contrasts with the consistent distal accumulation of Dachs on the apical surface (Mao et al., 2006; Rogulja et al., 2008) (Fig. S2). Hence, if Dachs directly influences Wts conformation, it appears to do so in a way that does not require a persistent association of asymmetrically distributed Dachs with a conformationally distinct form of Wts.
Wts conformation is regulated by Ft/Ds signaling
Heterodimeric, intercellular bridges between the protocadherins Ft and Ds generate signals that promote growth by repressing Wts kinase activity (Cho et al., 2006; Rogulja et al., 2008; Willecke et al., 2008; Zecca and Struhl, 2010). To determine if Ft/Ds signaling regulates the conformational state of Wts, we generated clones of mutant cells that cell-autonomously activate the Ft/Ds signal transduction pathway, or alternatively, that send strong, ectopic Ft or Ds signals to neighboring, wildtype cells, and assayed the effects on CWT sensor activity (Q).
(i) Clones lacking Ds and Ft
Removal of either Ds, Ft or both from otherwise wildtype wing cells results in cell autonomous activation of the Ft/Ds signal transduction pathway, which suppresses Wts activity (Bennett and Harvey, 2006; Cho et al., 2006; Matakatsu and Blair, 2006; Willecke et al., 2006; Zecca and Struhl, 2010). We observed that cells belonging to ft−, ds− or ds− ft− clones have higher Q values (appear red) relative to surrounding, wildtype cells (Figs. 3A, S3, and S4A). Hence, the mutant cells have an abnormally high proportion of Wts in the “closed” conformation, correlating with the reduced activity of Wts.
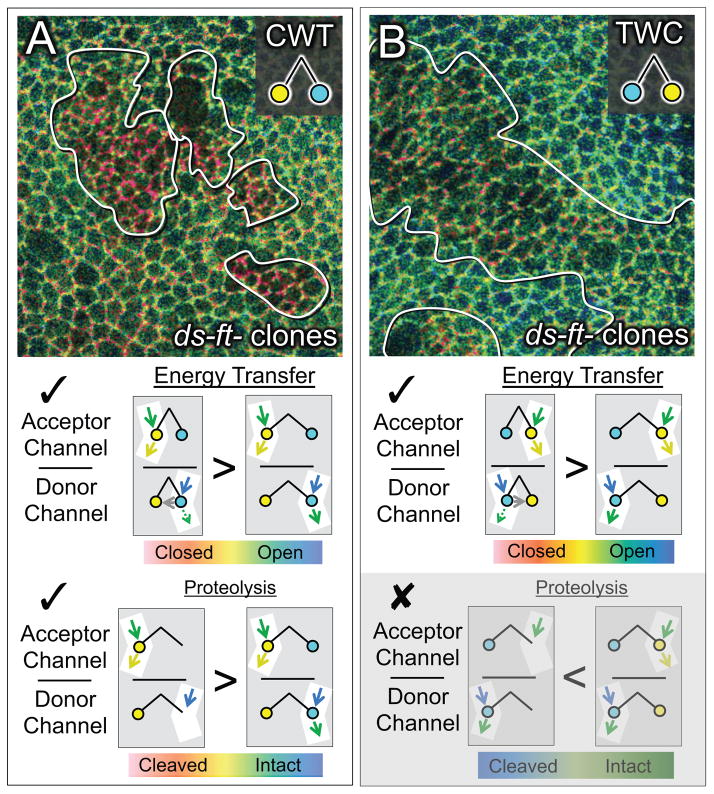
Figure 3. ds−ft− cells show increased Wts in the “closed” conformational state.
A) Q ratio image of wing tissue expressing the CWT sensor and containing clones of ds−ft− cells (outlined; Q ratio scale as in Fig. 2). Clones appear red relative to the wildtype surround. See Fig. S3 for Q ratio images of tissue containing single mutant ds− and ft− clones, and Fig. S4 for further examples and documentation of Q ratios and CWT abundance associated with ds−ft− cells relative to surrounding wildtype cells.
As diagrammed underneath, Q (the ratio of acceptor and donor channel emissions) is expected to rise (
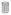 ) if the mutant condition causes an increase in either energy transfer or C-terminal proteolysis.
) if the mutant condition causes an increase in either energy transfer or C-terminal proteolysis.
B) Q ratio image for ds−ft− clones in wing tissue expressing the TWC sensor. As in A, clones appear red relative to the surround, as expected if energy transfer increases in the mutant cells (
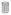 ). However, clones should appear blue in the case of C-terminal proteolysis, which was not observed (
). However, clones should appear blue in the case of C-terminal proteolysis, which was not observed (
 ; greyed out). Note that the difference between the clone and surround is less pronounced for the TWC than the CWT sensor (A), possibly reflecting a less favorable dipole orientation of the two fluorophores in the TWC sensor.
; greyed out). Note that the difference between the clone and surround is less pronounced for the TWC than the CWT sensor (A), possibly reflecting a less favorable dipole orientation of the two fluorophores in the TWC sensor.
Previous studies have shown that the loss of Wts activity in ft− cells correlates with a reduction in Wts protein levels (Cho et al., 2006). In agreement, we found that apical CWT protein levels in both ft− and ds−ft− clones, as detected in the acceptor channel, are sometimes reduced relative to surrounding wildtype cells (up to 40% lower, e.g. ds−ft− clones in Fig. S4C). Nevertheless, the remaining clones show little if any reduction in apical CWT accumulation (Fig. S4C), even though all ft− and ds−ft− clones show similar increases in Q relative to the surround (Figs. S3C, S3D, S4D, S4E). Hence, the increased occupancy of CWT in the “closed” state in such clones appears independent of the variably reduced levels of protein.
The reduction in CWT accumulation observed in some ft− and ds−ft− clones does, however, raise the possibility that the consistent increase in Q observed in all ft− and ds−ft− clones is due to proteolytic cleavage of the CWT sensor, rather than increased energy transfer. In particular, as depicted in Fig. 3A, a cleavage event that releases the C-terminal mTFP1 from its association with the rest of the protein at the apical cell membrane would cause an increase in Q (the ratio of acceptor to donor channel emission), even if the two fluorophores are never sufficiently close to allow energy transfer. To test this, we used TWC, a permuted Wts FRET sensor in which the positions of the two fluorophores are swapped (Fig. 3B). In this case, a corresponding cleavage that dissociates the now C-terminal mCitrine from the rest of the protein should result in a decrease rather than an increase in Q within ds−ft− clones (i.e., clones should appear bluer rather than redder than the surround). However, we still observed an increase in Q (Fig. 3B, clones appear redder), albeit to a lesser degree than observed for the CWT sensor (Fig. 3A), possibly due to a less favorable orientation of the two fluorophores for energy transfer in TWC. Despite the difference in degree, the fact that ds−ft− clones show an increase in Q for both TWC and CWT sensors indicates that the increase reflects a gain in proximity-dependent energy transfer rather than the loss of the C-terminal fluorophore.
ii) Clones ectopically expressing Ds or Ft
Ft and Ds interactions are modulated by the Golgi ecto-kinase, Four-jointed (Fj), which enhances the capacity of cells to send “Ft signal” and receive “Ds signal” (Brittle et al., 2010; Simon et al., 2010). In the wing disc, Ft expression is uniform, whereas Fj expression peaks in the center of the wing primordium and Ds expression peaks in the surrounding hinge primordium (Clark et al., 1995; Villano and Katz, 1995; Rogulja et al., 2008). These expression patterns result in elevated Ft/Ds signaling interactions between predominantly Ft (wing) and Ds (hinge) signaling cells at the wing periphery (Fig. 4) that recruit new cells into the wing primordium by locally suppressing Wts activity (Zecca and Struhl, 2010). Accordingly, clones of cells that overexpress Ds in the middle of the prospective wing create ectopic signaling interfaces with their wildtype, Ft signaling neighbors (Fig. 4A), whereas Ft over-expressing clones in the hinge create ectopic signaling interfaces with their wildtype, Ds signaling neighbors (Fig. 4B). Both kinds of signaling interfaces change the behavior of neighboring cells, inducing growth by locally suppressing Wts activity (Rogulja et al., 2008; Willecke et al., 2008; Zecca and Struhl, 2010) and redirecting planar cell polarity (Casal et al., 2002; Casal et al., 2006; Mao et al., 2006; Ambegaonkar et al., 2012; Brittle et al., 2012). We therefore asked if such ectopic Ft/Ds signaling causes a change in Wts conformation as monitored by the CWT sensor.
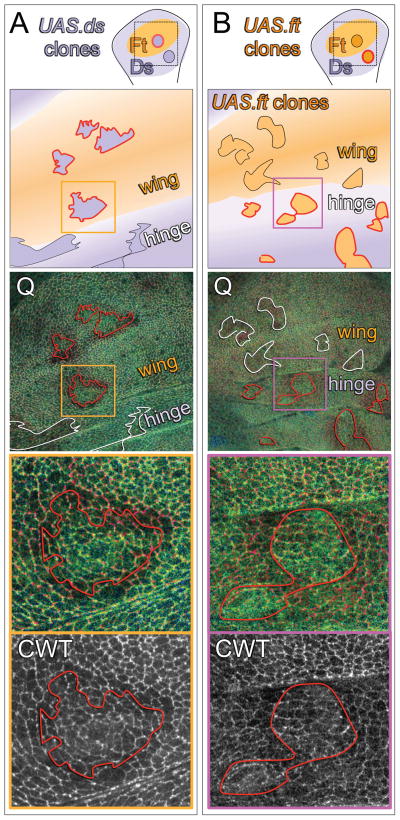
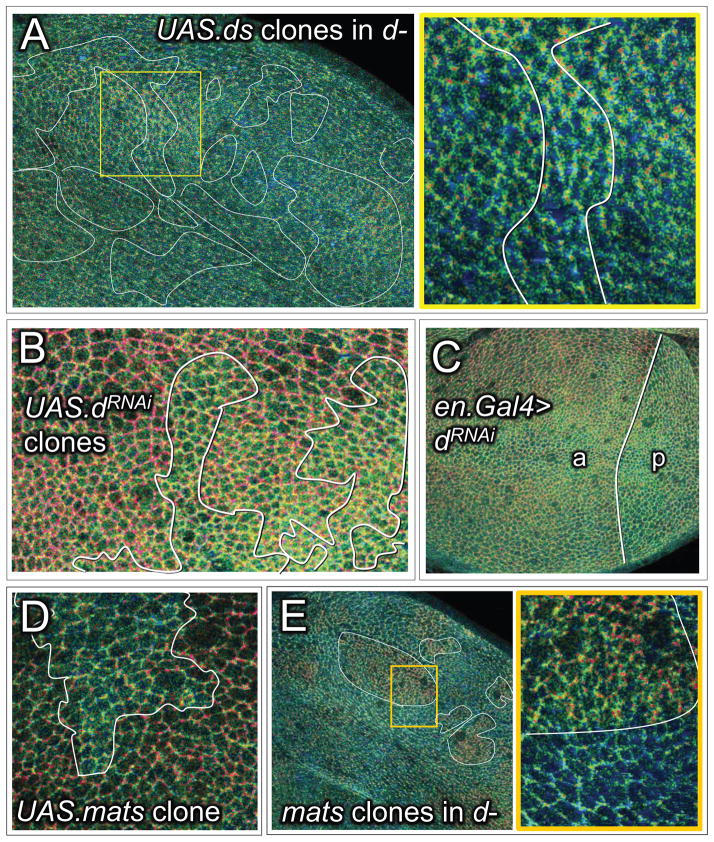
Figure 4. Ft/Ds signaling regulates the conformational state of Wts.
A, B) Wing discs containing UAS.ds (A) or UAS.ft (B) over-expressing clones. As in the cartoons, the wing and hinge primordia are shaded orange and purple, respectively, to indicate that they comprise predominately Ft or Ds signaling cell populations, and the clones are shown as darker orange or purple to indicate the over-expression of Ft or Ds protein. Clone borders that create abrupt, ectopic confrontations between Ft and Ds signaling cells are outlined in red, and those that do not, are outlined in black (cartoons) or white (Q ratio images; Q ratio scale as in Fig. 2). UAS.ds clones (A) induce an increase in Q in surrounding wildtype cells (appear red) when located in the wing, but not in the hinge. Conversely, UAS.ft clones (B) induce an increase in Q when located in the hinge, but not in the wing. Q ratio and CWT (acceptor channel) images of the regions boxed in orange and purple in the middle panels are shown at higher magnification in the bottom panels: clones that create abrupt, ectopic signaling interfaces induce higher Q values in wildtype cells up to four cell diameters away.
We find that clones that over-express Ds are associated with local, non-autonomous increases in Q when located in central portions of the wing primordium, where they create steep, ectopic Ds/Ft signaling confrontations (as indicated by increased “redness” in cells neighboring the clone border; Fig. 4A). Note that Q is higher on both sides of the borders of such clones, consistent with prior evidence that Wts activity is locally suppressed on both sides (Rogulja et al., 2008; Willecke et al., 2008). In contrast, no such increases in Q were detected in the hinge primordium, where the clones and the surround have similar Ds sending and Ft receiving properties (Fig. 4A). Conversely, Ft overexpressing clones (Fig. 4B) induce local non-autonomous increases in Q in the prospective hinge territory, where they should create steep Ft/Ds signaling confrontations, but not in the prospective wing region, where they should not (Fig. 4B).
Thus, the conformational state of Wts correlates with the confrontational intensity of Ft/Ds signaling. When the intensity is high, more Wts molecules adopt the “closed” state; when low, no enrichment of “closed” Wts is observed. Taken together with the transition of Wts from the “open” to “closed” state in ds−, ft−, and ds−ft− clones, which constitutively activate the Ft/Ds signal transduction pathway, these findings indicate that Ft/Ds signaling regulates the conformation of Wts.
Ft/Ds signaling acts via Dachs to regulate Wts conformation
Ft/Ds signaling acts through the agency of the atypical myosin Dachs to suppress Wts activity. In the absence of Dachs, Wts activity is not suppressed, even under conditions that create abrupt confrontations between Ft and Ds signaling cells such as depicted in Figure 4 (Mao et al., 2006; Rogulja et al., 2008; Zecca and Struhl, 2010). To determine if Ft/Ds signaling regulates the conformation of Wts via Dachs, we repeated the experiments shown in Figure 4 in dachs− (d−) mutant discs.
In the absence of ectopic Ds or Ft expressing clones, Q ratio images of d− discs appear predominantly green, as expected if Dachs is essential for the normal central-to-peripheral shift in Wts from the “open” to the “closed” state. The same is also true for d− discs that contain Ds or Ft over-expressing clones (e.g., Fig. 5A), even though such clones induce non-autonomous increases in Q in the wing and hinge primordia, respectively, in otherwise wildtype discs (Fig. 4). Thus, the conformational state of Wts in entirely d− discs appears impervious to Ft/Ds signaling, arguing that these signals act through the agency of Dachs to regulate Wts conformation.
Figure 5. Ft/Ds signaling acts via Dachs to inhibit or reverse the “closed” to “open” transition of Wts mediated by Mats.
A) Q ratio image of a d− wing disc containing numerous, large UAS.ds over-expressing clones: the yellow box includes a clone located within the prospective wing, shown at higher magnification underneath (Q ratio scale for A–E as in Fig. 2). In contrast to wt discs, d− discs appear predominantly green and clones of UAS.ds over-expressing cells in such discs fail to induce an increase in Q in surrounding cells (compare with Fig. 4A), indicating general occupancy of the CWT sensor in the “open” state, irrespective of Ft/Ds signaling.
B) Q ratio image of wing tissue containing clones of cells in which Dachs activity has been knocked down by UAS.dRNAi expression. Clones appear green relative to the surround. Similar results were obtained for clones of homozygous d− cells, although it was necessary either to over-express Yki in such clones, or to generate them in wing discs with reduced ex gene function (Fig. S7), to allow the d− cells to grow similarly to surrounding cells that retain normal d function.
C) Q ratio image of a wing disc in which Dachs activity has been knocked down selectively in the posterior (p) compartment by expressing UAS.dRNAi under en.Gal4/UAS control. The posterior compartment (p), which is abnormally small, is predominantly green/blue. In contrast, the anterior (a) compartment shows the normal central-to-peripheral, green-to-red shift.
D) Q ratio image of proximal wing tissue containing a clone of UAS.mats over-expressing cells. The clone appears green relative to the surround, indicating increased occupancy of the CWT sensor in the “open” state.
E) Q ratio image of a d− wing disc with several matse03077 clones (some outlined in white); a portion of one such clone, boxed in yellow, is shown at higher magnification to the right. The clones appear red, in contrast to the surround, which appears blue-green, indicating that occupancy of the CWT sensor in the “open” state in d− cells depends on Mats.
To assess the effects of reducing Dachs activity in defined sub-populations of cells relative to neighboring wildtype cells, we performed two experiments. First, we assayed Q in clones of cells in which Dachs expression was knocked down by RNAi. Such clones appear green/blue in Q ratio images, contrasting with surrounding wildtype cells, which appear redder, particularly at the wing periphery (Fig. 5B). Similar results were obtained with clones of homozygous d− clones (Fig. 5 legend and Fig. S7). Second, we assayed discs in which Dachs expression was selectively reduced by RNAi in the posterior compartment (Fig. 5C). The posterior compartments of such discs are abnormally small owing to excess Wts activity, and appear predominantly green in contrast to the anterior compartments which show the normal green-to-red, central-to-peripheral shift (Fig. 5C). These results confirm that Dachs is required to promote or stabilize the “closed” state of Wts, as expected if Dachs acts downstream of Ft/Ds signaling to suppress Wts activity.
Dachs regulates Wts conformation by inhibiting or reversing the action of Mats
As described above, Mats deficient clones have constitutively high Q values (appear red) whereas Dachs deficient clones have constitutively low Q values (appear green), indicating that Mats and Dachs have opposing effects on Wts conformation (Fig. 1A). In agreement, clones of cells that overexpress Mats at the wing periphery appear green relative to the surrounding cells, suggesting that excess Mats can counteract the effect of Dachs on Wts (Fig. 5D).
To assess the nature of the opposing actions of Mats and Dachs on Wts, we assayed Q in clones of matse03077 cells in d− discs. We found that the d− matse03077 cells in such clones appear red relative to the surrounding green d− tissue (Fig. 5E), indicating that Wts cannot adopt the “open” state in the absence of Mats, even when Dachs is not present to oppose the ”closed” to “open” transition. Accordingly, we infer that Mats is normally required for Wts to adopt the “open” conformation and that Dachs transduces Ft/Ds signaling by blocking or reversing this transition.
Regulation of Wts conformation by Ft/Ds/D signaling is independent of Wts phosphorylation by Hpo
Hpo family kinases phosphorylate NDR kinases at a conserved threonine residue, T1083 (http://flybase.org/), within the hydrophobic motif of Wts (Chan et al., 2005). This phosphorylation event is essential for Wts kinase activity. To assess if phosphorylation by Hpo causes a change in Wts conformation that can be detected by our CWT sensor, we performed the following experiments.
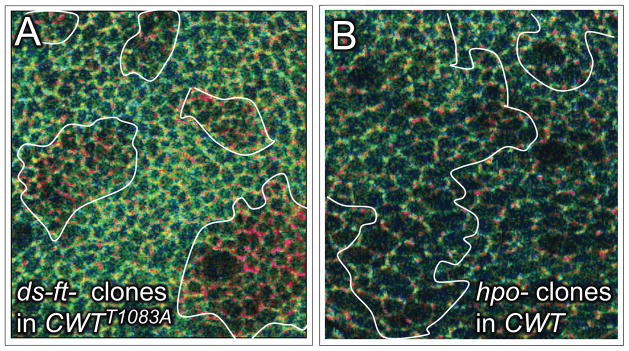
First, we generated CWTT1083A, a CWT sensor in which Threonine1083 is replaced by Alanine. CWTT1083A is functionally null; hence we compared its sensor activity relative to wildtype CWT in animals that carried a single wildtype copy of the endogenous wts gene. We find that the CWTT1083A sensor behaves similarly to the wildtype CWT sensor in wts−/+ heterozygotes and wts− homozygotes in terms of its abundance, subcellular localization and the characteristic central-to-peripheral increase in Q, visualized as a green-to-red shift in Q ratio images. Further, the CWTT1083A sensor shows a similar, cell-autonomous increase in Q in ds−ft− clones (Fig. 6A; clones appear red relative to the surround). Thus, it appears that Ft/Ds signaling regulates the conformational state of the mutant sensor as it does the wildtype sensor, even though the mutant sensor cannot be phosphorylated at T1083.
Figure 6. Wts conformational state is independent of Hpo phosphorylation.
A) Q ratio image of wing tissue expressing the mutant CWTT1083A sensor and containing clones of ds−ft− cells (Q ratio scale for A and B as in Fig. 2). Clones appear red relative to the wildtype surround, indicating an increase in occupancy in the “closed” state, as observed for the wildtype CWT sensor (Fig. 3A; Fig. S4) even though the sensor cannot be phosphorylated by Hpo.
B) Q ratio image of hpo− clones within the prospective wing. No significant difference in Q between mutant and wildtype tissue is apparent, even though the CWT sensor cannot be phosphorylated by Hpo within the clone. See Fig. S5 for a low magnification image of the disc containing the clones in this image (Fig. S5B) as well as two other discs that provide examples of different size hpo− clones, all of which show no significant increase in Q relative to the wildtype surround. Note that Fig. S5 offers examples of ex− clones, and Fig. S6 shows that RNAi knock-down of Mats and Ft, but not of Hpo or Ex, causes an increase in CWT sensor activity (Q).
Second, we assayed the response of the wildtype sensor in clones of cells homozygous for hpo5.1, a null allele (Genevet et al., 2009), in which all CWT protein should be unphosphorylated at T1083. In accord with the results obtained with the CWTT1083A sensor, we failed to detect any difference in Q between cells in hpo− clones versus surrounding wildtype cells (Fig. 6B; Fig. S5A–C). As a further test, we assayed Q in clones devoid of Expanded (Ex), a FERM domain protein required for Hpo activity (Hamaratoglu et al., 2006) and found that these also show no increase in Q (Fig. S5D, E). These findings contrast with the results of generating clones lacking Mats, Ds, Ft, or both Ds and Ft, all of which show readily detectable cell-autonomous increases in Q (Figs. 1D, 3A; Figs. S3,4), and are corroborated by experiments comparing the effects of RNAi knockdown of Hpo, Ex, Ft and Mats. Knock-down of each of these functions results in wing over growth, indicating that RNAi was effective, but only Ft and Mats knockdown, and not Hpo or Ex knockdown, result in an increase in Q (Fig. S6).
Thus, Wts conformation, as monitored by the CWT sensor, does not appear to depend on the phosphorylation of the T1083 residue targeted by Hpo kinase, nor on Hpo kinase activity.
Significantly, our results argue against both direct and indirect effects of Hpo kinase activity on the Mats-dependent transition of Wts from the “closed” to “open” state, and in particular, on the possibility that Hpo phosphorylation of Mats is essential for Mats to interact with Wts (Wei et al., 2007; Praskova et al., 2008; Ni et al., 2015). If so, Q would be elevated in hpo− clones (appear red relative to the surround), because unphosphorylated Mats would not be able to bind to Wts and mediate the transition. Further, we generated clones of d− cells in animals homozygous for a hypomorphic allele of ex, ex697, in which all cells have reduced Hpo kinase activity. Such d− clones nevertheless exhibit lower Q (appear green relative to the surround; Fig. S7). Given that the decrease in Q caused by the removal of Dachs depends on Mats (Fig. 5E), we infer that Mats is able to mediate the “closed” to “open” transition despite the reduction in Ex-dependent Hpo activity.
Finally, we tested if the conformational transition monitored by CWT depends on Wts activation loop phosphorylation (possibly by auto-phosphorylation), a key step in Wts kinase activation. To do so, we assayed Q for two additional mutant sensors: (i) CWTK749R, a “kinase dead” form in which a conserved Lysine essential for NDR kinase activity (Xia et al., 2002) is mutated to Arginine, and (ii) CWTS920A, in which the conserved activation segment Serine implicated in auto-phosphorylation (Hergovich et al., 2006b) is mutated to Alanine. As expected, both forms are devoid of Wts rescuing activity. Nevertheless, when assayed for energy transfer in animals carrying a single, wildtype copy of the endogenous gene, both behaved like wildtype CWT, e.g., as indicated by the abrupt increase in Q observed in ds−ft− clones (clones appear red, Fig. S8). Hence, the transition of Wts from the “closed” to “open” state does not appear to depend on auto-phosphorylation.
Taken together, these results indicate that regulation of Wts conformation by Mats, Ft/Ds signaling and Dachs, as detected by our CWT sensor, occurs independently of the regulation of Wts by Hpo phosphorylation or Wts auto-phosphorylation, establishing that Wts is governed by mechanistically distinct allosteric and phosphorylation mechanisms.
Discussion
NDR kinases comprise the central components of a basic transducing module that regulates tissue growth in animals [reviewed in (Pan, 2010; Staley and Irvine, 2012; Enderle and McNeill, 2013; Hergovich, 2013)]. Kinase activity imposes a general brake on growth by phosphorylating conserved transcriptional co-activators such as Drosophila Yki and mammalian YAP/TAZ, blocking their access to the nucleus (Huang et al., 2005; Hao et al., 2008). Hence, growth depends on the capacity of cells to down-regulate NDR kinase activity, e.g., in response to global organizing signals, local cell-cell interactions, or mechanical tension. NDR kinases must be bound by Mob family proteins and phosphorylated by Hpo family kinases to be active (Pan, 2010; Staley and Irvine, 2012; Enderle and McNeill, 2013; Hergovich, 2013). To date, most inputs that suppress NDR kinases appear to do so by inhibiting or reversing their phosphorylation by Hpo kinases. Here, we identify an input that blocks or countermands the action of Mob proteins and identify an allosteric mechanism by which these proteins activate NDR kinases.
Activation of NDR kinases requires Mob protein dependent, conformational change
Mob family proteins bind NDR kinases through conserved residues, and binding is required for NDR kinase auto-phosphorylation and activity (Bichsel et al., 2004; He et al., 2005; Hergovich et al., 2006a). It has been suggested that Mob binding allows recruitment of other factors necessary for an active NDR kinase complex, or possibly to drive NDR kinases from a “closed”, auto-inhibited state to an “open”, potentially active state (Bichsel et al., 2004; Lai et al., 2005). Here, we have sought to determine how the Drosophila NDR kinase Wts is activated by the Mob protein Mats. Using a genetically encoded, Wts-FRET sensor to monitor interactions between the Wts amino- and carboxy-termini in vivo, we find that association between the two ends of the kinase correlates with the loss of kinase activity. Further, by removing Mats, or using a mutant Wts-FRET sensor that cannot be bound by Mats, we find evidence for a causal relationship between Mats binding, the conformational state of Wts, and Wts kinase activity, namely, that Mats binding is required for Wts to undergo a conformation transition from a “closed” to an “open” state upon which kinase activity depends (Fig. 1A).
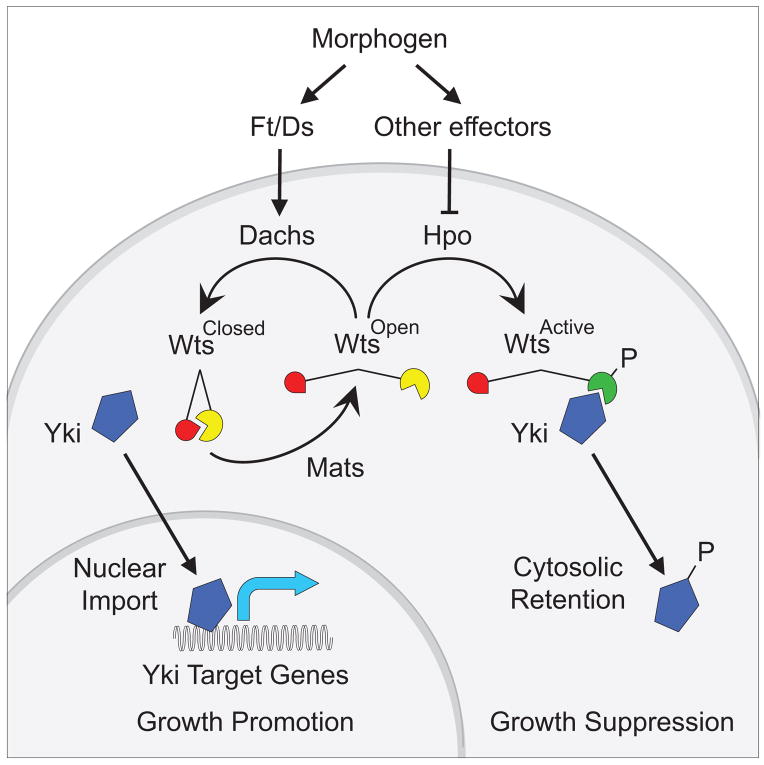
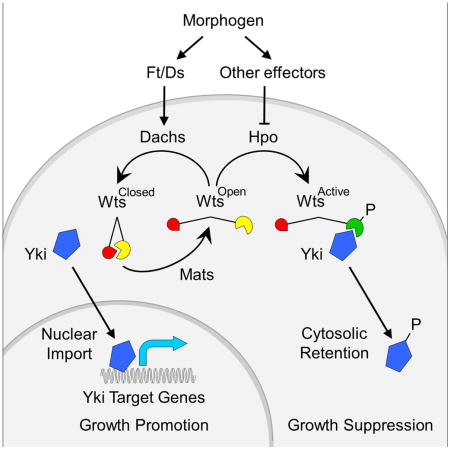
Importantly, the Mats-dependent conformational change of Wts detected by the Wts-FRET sensor does not appear to depend on phosphorylation of Wts by Hpo kinase. Removing either Hpo kinase activity or the capacity of the Wts-FRET sensor to be phosphorylated by Hpo has no effect on energy transfer, even though both manipulations abolish Wts kinase activity. Likewise, blocking the capacity of Wts to auto-phosphorylate itself abolishes Wts kinase activity without affecting the “closed” to “open” transition mediated by Mats. Hence, Mats appears to regulate Wts by an allosteric mechanism that is distinct from the regulation of Wts by phosphorylation. In principle, the two mechanisms may operate independently, neither being required for the other to occur, but both being equally essential for Wts kinase activity. However, the removal of Mob protein activity from mice severely compromises phosphorylation of Lats1 kinase by the Hpo kinases Mst1/2 (Nishio et al., 2012), suggesting that the two mechanisms operate in sequence, with NDR kinases first having to undergo the Mob-dependent conformational change to be phosphorylated by Hpo kinases (Fig. 7).
Figure 7. Two step model for the activation of Wts.
Activation of Wts requires a “closed” to “open” conformational transition mediated by Mats, followed by phosphorylation of the “open” form by Hpo. Morphogen input regulates the first step via Ft/Ds signaling and Dachs to inhibit or reverse the action of Mats, and possibly the second step via other effectors that control the phosphorylation of Wts by Hpo (e.g. by altering Wts localization or stability; not depicted). The level of Wts activity governs the balance between cytosolic retention and nuclear access of Yki and hence the expression of Yki target genes that control growth.
We note that there is evidence that Hpo kinases can phosphorylate Mob proteins to initiate additional phosphorylation and conformational events involving Mob, Hpo and NDR proteins within a Mob/Hpo/NDR complex (Wei et al., 2007; Praskova et al., 2008; Ni et al., 2015). We suggest that if these additional events are relevant in Drosophila wing cells, they occur downstream of the Mats-dependent conformational transition detected by our Wts-FRET sensor.
Fat/Dachsous signaling and Dachs regulate Wts activity by inhibiting or reversing Mats-dependent conformation change
Previously, most regulatory inputs into the control of NDR kinase activity have been attributed to mechanisms that regulate their phosphorylation by Hpo kinases or their stability (Pan, 2010; Halder et al., 2012; Staley and Irvine, 2012; Enderle and McNeill, 2013; Hergovich, 2013). However, we find that the protocadherins Fat (Ft) and Dachsous (Ds) control Wts activity by a different mechanism in the Drosophila wing, by regulating its Mats-dependent conformational state via the unconventional myosin Dachs.
In most developing Drosophila epithelia, morphogens such as Wg, Dpp and Hh generate opposing differentials of Ft and Ds signaling activities (Casal et al., 2002; Yang et al., 2002a; Casal et al., 2006; Rogulja et al., 2008; Zecca and Struhl, 2010), resulting in oriented, asymmetric accumulation of Dachs (Mao et al., 2006; Ambegaonkar et al., 2012; Bosveld et al., 2012; Brittle et al., 2012). Our results provide three insights into possible mechanisms by which Ft/Ds signaling and the asymmetric accumulation of Dachs suppress Wts activity to promote growth.
First, the primary effect of Dachs on Wts is to inhibit or reverse the Mats-dependent conformational change. This could occur by direct action of Dachs, e.g., to block the binding of Mats to Wts or prevent the putative “closed” to “open” transition mediated by Mats. Alternatively, Dachs could mediate a competing and opposing “open” to “closed” transition, thus titrating the availability of “open” Wts for phosphorylation by Hpo. AsDachs is localized predominately at the apical membrane (Mao et al., 2006; Ambegaonkar et al., 2012; Bosveld et al., 2012; Brittle et al., 2012) while Mats is present more generally in the cytosol [(Ho et al., 2010); our unpublished observations], Wts may shuttle between subcellular domains where it is “opened” and “closed”, respectively, by Mats and Dachs.
Second, occupancy of Wts in the “closed” state correlates with both suppression of Wts activity and the degree of subcellular Dachs asymmetry. Previously, we showed that Wts activity, under Ft/Ds/Dachs control, is not uniform in the developing wing, but rather is locally suppressed at the wing periphery to recruit neighboring cells into the wing primordium (Zecca and Struhl, 2010); others have shown that the extent of Dachs asymmetry is likewise not uniform, but peaks at the periphery (Brittle et al., 2012; Merkel et al., 2014), where Wts is suppressed. Hence, the degree of Dachs subcellular asymmetry may control the occupancy of Wts in the “closed” vs the “open” state.
Third, and most intriguingly, we did not observe a spatial correlation within cells between the asymmetric accumulation of Dachs and the conformational state of Wts. Specifically, cells at the wing periphery show increased apical membrane microdomains with Wts in the “closed” state compared to cells located more centrally. However there is no apparent bias in the proximo-distal axis, in contrast to the enhanced, preferential accumulation of Dachs along the distal edge of each cell. Thus, the effect of Dachs on the conformational state of Wts is either indirect or does not require a persistent association between the two proteins.
Control of organ growth by morphogen-dependent suppression of NDR kinase activity
Growth of the Drosophila wing is governed by the morphogens Wg and Dpp. Wg and Dpp act at least in part by suppressing Wts (Rogulja et al., 2008; Zecca and Struhl, 2010). However, despite the many, diverse inputs that are known to regulate Wts in the wing, the only known pathway that links morphogen to the control of Wts is Ft/Ds signaling transduced by Dachs (Rogulja et al., 2008; Zecca and Struhl, 2010).
Previously, we have presented evidence for a model in which morphogen operates via Ft/Ds signaling and Dachs to locally suppress Wts at the wing periphery, leading to an increase of Yki activity that acts together with morphogen to recruit cells into the wing primordium (Zecca and Struhl, 2010). In accord, we find a central-to-peripheral gradient in the Ft/Ds/Dachs dependent conformation of Wts, with a peak in the “closed” state at the wing periphery. Further, abrupt, experimentally-induced disparities in Ft/Ds signaling cause Wts to adopt the “closed” state in neighboring cells, correlating with elevated wing growth and recruitment (Rogulja et al., 2008; Willecke et al., 2008; Zecca and Struhl, 2010). Thus, our findings link morphogen to wing growth specifically through the actions of Ft/Ds signaling and Dachs on Wts conformation.
Importantly, removal of Ft/Ds/Dachs input into Wts, by ablating Dachs, greatly reduces but does not abolish morphogen-dependent wing growth (Mao et al., 2006; Zecca and Struhl, 2010), suggesting that morphogen also suppresses Wts activity by other effectors (Fig. 7). Potential mechanisms include the regulation of Wts stability, its phosphorylation by Hpo, and its access to target proteins.
Morphogens have also been proposed to regulate organ growth by generating differentials in mechanical tension (Shraiman, 2005; Aegerter-Wilmsen et al., 2007; Hufnagel et al., 2007). Strikingly, both mathematical modeling and empirical tests indicate that mechanical tension in the developing wing increases in a central-to-peripheral fashion similar to what we observe for the Ft/Ds/Dachs-dependent transition of Wts from the “open” to the “closed” state (Nienhaus et al., 2009; Legoff et al., 2013). However, loss of Wts activity in cell clones causes a local, non-autonomous increase in mechanical tension in surrounding, wing tissue (Mao et al., 2013) — a result that contrasts with our finding that mats− and ds− ft− clones, both of which result in a loss of Wts activity, have a cell autonomous effect on our Wts-FRET sensor. Assuming that such clones behave like wts− clones in increasing mechanical strain in surrounding tissue, their failure to influence Wts-FRET sensor activity in neighboring cells argues that mechanical tension does not regulate the Mats-dependent conformational state of Wts.
In sum, we now extend a causal progression from morphogen to Ft/Ds/D signaling (Zecca and Struhl, 2010) to the control of wing growth via regulation of the conformational state of Wts – a distinct step that we suggest precedes and is required for the phosphorylation of Wts by Hpo (Fig. 7). This allosteric mechanism may be integrated with others such as the control of Hpo-dependent phosphorylation of Wts or Wts stability to achieve the appropriate amount of Wts activity throughout the developing wing. We posit that the control of Wts conformational state reflects the first step of a potentially general two-step mechanism of NDR kinase activation by which NDR kinases may be regulated.
Experimental Procedures
Transgenes
The coding sequences for monomeric Citrine (mCitrine) and monomeric Teal (mTFP1) (Ai et al., 2008), were fused to the 5′ and 3′ ends, respectively, of the wild type, R708A, K749A, S920A, and T1083 Wts coding sequences to encode wild type and mutant forms of “CWT”, a Wts-FRET sensor, and at 3′ and 5′ ends of the wild type coding sequence to encode “TWC”, a permuted Wts-FRET sensor. The CWT and TWC sensors were expressed uniformly and at physiological levels, in the developing wing imaginal disc using the Tubulinα1 (Tubα1) and ribosomal protein 49 (rp49) promoters, or in clones of Tubα1>CWT cells generated in Tubα1>stop>CWT discs using the Flp-out technique (Struhl and Basler, 1993). See Extended Experimental Methods for details.
Generation and analysis of mutant tissue
Clones marked by expression of myristoylated RFP were generated by standard Flp-out and Flp-mediated mitotic recombination methods (Golic, 1991; Struhl and Basler, 1993), including the MARCM technique (Lee and Luo, 1999); see Extended Experimental Methods for exact genotypes. Larvae of the appropriate genotype were heat shocked at 35°C for 15 minutes to produce Tubα1>CWT Flp-out clones or 36°C for 30 minutes to induce Flp-mediated mitotic recombination. Third instar larvae were dissected in PBS, and immediately fixed (2% formaldehyde PBS, 30 minutes; PBS rinse, 30 minutes). Wing discs were mounted in Prolong media without anti-fade (Invitrogen, P-7481) and oriented with the apical surface of the columnar epithelium facing up.
Images of the prospective wing and adjoining dorsal wing hinge (Fig. 2A, dashed box area) were acquired as Z stacks (0.29 nm intervals) using an upright Leica SP5 confocal microscope (40X 1.25 NA oil objective). For quantitative measurements we averaged the donor, FRET, and acceptor channel signals (Fig. 1B, C) for CWT localized in apical membrane enrichments along each of 30 randomly sampled cell-cell interfaces. We then subtracted average background intensities, as determined under identical imaging conditions for a wing disc that does not expresses CWT, and computed R and Q as defined in the text and Fig. 1B, C, and shown in Fig. 1E.
Supplementary Material
Acknowledgments
We thank S. Kidd, T. Lieber, M. Zecca, J. Parker, A. Tomlinson and I. Greenwald for comments on the manuscript. We thank S. Kameoka for the ds-mCherry and ft-mCherry transgenes and N. Tapon, the Bloomington stock center, and the Vienna Drosophila RNAi Center for stocks. We gratefully acknowledge financial support from the Jane Coffin Childs Fund (A.M.V.), the Howard Hughes Medical Institute and NIH RO1 GM113000 (G.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aegerter-Wilmsen T, Aegerter CM, Hafen E, Basler K. Model for the regulation of size in the wing imaginal disc of Drosophila. Mech Dev. 2007;124:318–326. doi: 10.1016/j.mod.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Ai HW, Hazelwood KL, Davidson MW, Campbell RE. Fluorescent protein FRET pairs for ratiometric imaging of dual biosensors. Nat Methods. 2008;5:401–403. doi: 10.1038/nmeth.1207. [DOI] [PubMed] [Google Scholar]
- Ambegaonkar AA, Pan G, Mani M, Feng Y, Irvine KD. Propagation of Dachsous-Fat Planar Cell Polarity. Curr Biol. 2012 doi: 10.1016/j.cub.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Bichsel SJ, Tamaskovic R, Stegert MR, Hemmings BA. Mechanism of activation of NDR (nuclear Dbf2-related) protein kinase by the hMOB1 protein. J Biol Chem. 2004;279:35228–35235. doi: 10.1074/jbc.M404542200. [DOI] [PubMed] [Google Scholar]
- Bosveld F, Bonnet I, Guirao B, Tlili S, Wang Z, Petitalot A, Marchand R, Bardet P-L, Marcq P, Graner F, et al. Mechanical Control of Morphogenesis by Fat/Dachsous/Four-Jointed Planar Cell Polarity Pathway. Science. 2012 doi: 10.1126/science.1221071. [DOI] [PubMed] [Google Scholar]
- Brittle A, Thomas C, Strutt D. Planar Polarity Specification through Asymmetric Subcellular Localization of Fat and Dachsous. Current Biology. 2012:1–8. doi: 10.1016/j.cub.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittle AL, Repiso A, Casal J, Lawrence PA, Strutt D. Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr Biol. 2010;20:803–810. doi: 10.1016/j.cub.2010.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RE. Fluorescent-protein-based biosensors: modulation of energy transfer as a design principle. Analytical chemistry. 2009;81:5972–5979. doi: 10.1021/ac802613w. [DOI] [PubMed] [Google Scholar]
- Casal J, Lawrence PA, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J, Struhl G, Lawrence PA. Developmental compartments and planar polarity in Drosophila. Curr Biol. 2002;12:1189–1198. doi: 10.1016/s0960-9822(02)00974-0. [DOI] [PubMed] [Google Scholar]
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine K. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- Clark HF, Brentrup D, Schneitz K, Bieber A, Goodman C, Noll M. Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev. 1995;9:1530–1542. doi: 10.1101/gad.9.12.1530. [DOI] [PubMed] [Google Scholar]
- Enderle L, McNeill H. Hippo gains weight: added insights and complexity to pathway control. Science signaling. 2013;6:re7. doi: 10.1126/scisignal.2004208. [DOI] [PubMed] [Google Scholar]
- Genevet A, Polesello C, Blight K, Robertson F, Collinson LM, Pichaud F, Tapon N. The Hippo pathway regulates apical-domain size independently of its growth-control function. J Cell Sci. 2009;122:2360–2370. doi: 10.1242/jcs.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golic KG. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- He Y, Emoto K, Fang X, Ren N, Tian X, Jan YN, Adler PN. Drosophila Mob family proteins interact with the related tricornered (Trc) and warts (Wts) kinases. Mol Biol Cell. 2005;16:4139–4152. doi: 10.1091/mbc.E05-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A. Regulation and functions of mammalian LATS/NDR kinases: looking beyond canonical Hippo signalling. Cell & bioscience. 2013;3:32. doi: 10.1186/2045-3701-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A, Schmitz D, Hemmings BA. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochemical and biophysical research communications. 2006a;345:50–58. doi: 10.1016/j.bbrc.2006.03.244. [DOI] [PubMed] [Google Scholar]
- Hergovich A, Stegert MR, Schmitz D, Hemmings BA. NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol. 2006b;7:253–264. doi: 10.1038/nrm1891. [DOI] [PubMed] [Google Scholar]
- Ho LL, Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated at the cell membrane to control tissue growth and organ size in Drosophila. Dev Biol. 2010;337:274–283. doi: 10.1016/j.ydbio.2009.10.042. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hufnagel L, Teleman AA, Rouault H, Cohen SM, Shraiman BI. On the mechanism of wing size determination in fly development. Proc Natl Acad Sci USA. 2007;104:3835–3840. doi: 10.1073/pnas.0607134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- Lacowicz JR. The Principles of Fluorescence Spectroscopy. 3. Springer Science + Business Media, LLC; New York: 2006. [Google Scholar]
- Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Lawrence PA, Struhl G. Morphogens, compartments, and pattern: lessons from drosophila? Cell. 1996;85:951–961. doi: 10.1016/s0092-8674(00)81297-0. [DOI] [PubMed] [Google Scholar]
- Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- Legoff L, Rouault H, Lecuit T. A global pattern of mechanical stress polarizes cell divisions and cell shape in the growing Drosophila wing disc. Development. 2013;140:4051–4059. doi: 10.1242/dev.090878. [DOI] [PubMed] [Google Scholar]
- Mao Y, Hoppe A, Kester L, Thompson BJ, Tournier AL, Tapon N. Differential proliferation rates generate patterns of mechanical tension that orient tissue growth. The EMBO Journal. 2013;32:2790–2803. doi: 10.1038/emboj.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Rauskolb C, Cho E, Hu WL, Hayter H, Minihan G, Katz FN, Irvine KD. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development. 2006;133:2539–2551. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- Merkel M, Sagner A, Gruber FS, Etournay R, Blasse C, Myers E, Eaton S, Jülicher F. The Balance of Prickle/Spiny-Legs Isoforms Controls the Amount of Coupling between Core and Fat PCP Systems. Current biology: CB. 2014;24:2111–2123. doi: 10.1016/j.cub.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Ni L, Zheng Y, Hara M, Pan D, Luo X. Structural basis for Mob1-dependent activation of the core Mst-Lats kinase cascade in Hippo signaling. Genes Dev. 2015;29:1416–1431. doi: 10.1101/gad.264929.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhaus U, Aegerter-Wilmsen T, Aegerter CM. Determination of mechanical stress distribution in Drosophila wing discs using photoelasticity. Mechanisms of development. 2009;126:942–949. doi: 10.1016/j.mod.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Nishio M, Hamada K, Kawahara K, Sasaki M, Noguchi F, Chiba S, Mizuno K, Suzuki SO, Dong Y, Tokuda M, et al. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. The Journal of clinical investigation. 2012;122:4505–4518. doi: 10.1172/JCI63735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18:311–321. doi: 10.1016/j.cub.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb C, Sun S, Sun G, Pan Y, Irvine KD. Cytoskeletal Tension Inhibits Hippo Signaling through an Ajuba-Warts Complex. Cell. 2014;158:143–156. doi: 10.1016/j.cell.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogulja D, Rauskolb C, Irvine KD. Morphogen Control of Wing Growth through the Fat Signaling Pathway. Dev Cell. 2008;15:309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shraiman BI. Mechanical feedback as a possible regulator of tissue growth. Proc Natl Acad Sci USA. 2005;102:3318–3323. doi: 10.1073/pnas.0404782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MA, Xu A, Ishikawa HO, Irvine KD. Modulation of fat:dachsous binding by the cadherin domain kinase four-jointed. Curr Biol. 2010;20:811–817. doi: 10.1016/j.cub.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley BK, Irvine KD. Hippo signaling in Drosophila: recent advances and insights. Developmental dynamics: an official publication of the American Association of Anatomists. 2012;241:3–15. doi: 10.1002/dvdy.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegert MR, Hergovich A, Tamaskovic R, Bichsel SJ, Hemmings BA. Regulation of NDR protein kinase by hydrophobic motif phosphorylation mediated by the mammalian Ste20-like kinase MST3. Mol Cell Biol. 2005;25:11019–11029. doi: 10.1128/MCB.25.24.11019-11029.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- Tabata T, Takei Y. Morphogens, their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- Tao W, Zhang S, Turenchalk GS, Stewart RA, St John MA, Chen W, Xu T. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat Genet. 1999;21:177–181. doi: 10.1038/5960. [DOI] [PubMed] [Google Scholar]
- Tsien RY, Harootunian AT. Practical design criteria for a dynamic ratio imaging system. Cell calcium. 1990;11:93–109. doi: 10.1016/0143-4160(90)90063-z. [DOI] [PubMed] [Google Scholar]
- Villano JL, Katz FN. four-jointed is required for intermediate growth in the proximal-distal axis in Drosophila. Development. 1995;121:2767–2777. doi: 10.1242/dev.121.9.2767. [DOI] [PubMed] [Google Scholar]
- Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J. 2007;26:1772–1781. doi: 10.1038/sj.emboj.7601630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen CL, Tao C, Zhang X, Halder G. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Willecke M, Hamaratoglu F, Sansores-Garcia L, Tao C, Halder G. Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0805201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Qi H, Li Y, Pei J, Barton J, Blackstad M, Xu T, Tao W. LATS1 tumor suppressor regulates G2/M transition and apoptosis. Oncogene. 2002;21:1233–1241. doi: 10.1038/sj.onc.1205174. [DOI] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Yang CH, Axelrod JD, Simon MA. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002a;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- Yang J, Cron P, Thompson V, Good VM, Hess D, Hemmings BA, Barford D. Molecular mechanism for the regulation of protein kinase B/Akt by hydrophobic motif phosphorylation. Mol Cell. 2002b;9:1227–1240. doi: 10.1016/s1097-2765(02)00550-6. [DOI] [PubMed] [Google Scholar]
- Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial Organization of Hippo Signaling at the Plasma Membrane Mediated by the Tumor Suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Struhl G. Control of Drosophila wing growth by the vestigial quadrant enhancer. Development. 2007;134:3011–3020. doi: 10.1242/dev.006445. [DOI] [PubMed] [Google Scholar]
- Zecca M, Struhl G. A feed-forward circuit linking wingless, fat-dachsous signaling, and the warts-hippo pathway to Drosophila wing growth. PLoS Biol. 2010;8:e1000386. doi: 10.1371/journal.pbio.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.