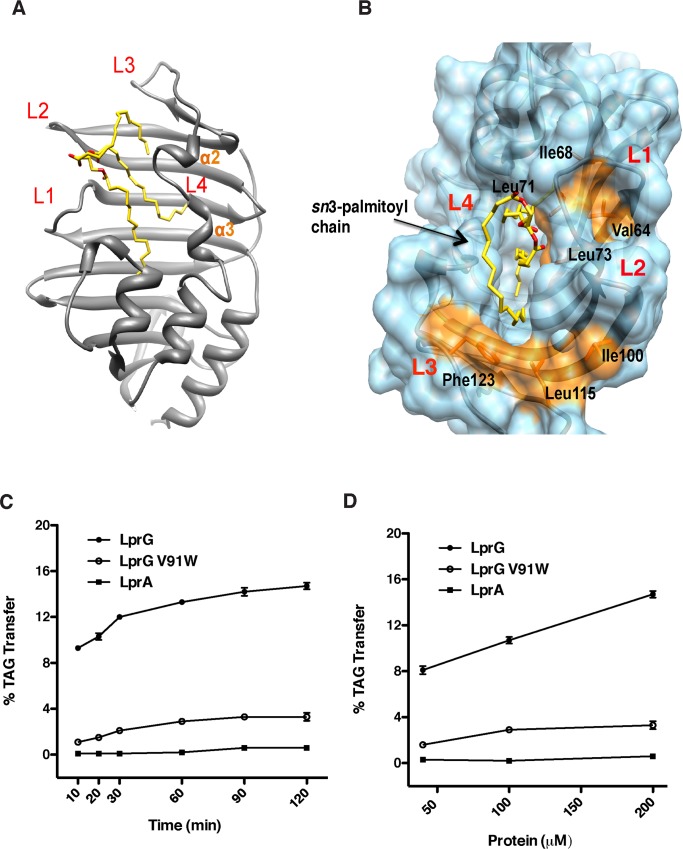
Fig 2. LprG binds and transports TAG in vitro.
(A) Structure of TAG (48:0) co-crystallized with LprG showing loops L1-L4 that surround the entrance to the binding cavity. (B) Two hydrophobic grooves (orange) on the surface of LprG and close to the binding site for the sn3-palmitoyl chain may accommodate TAG with longer acyl chains. (C) Addition of LprG (filled circles) to vesicles with partially self-quenched dye-labeled TAG results in a (C) time-dependent and (D) protein dose-dependent fluorescence increase. Controls include LprG with a bulky mutation V91W in the binding pocket and the homologue LprA. LprG-dependent transfer activity is apparent at the first time point (10 min.) due to lag time between reaction initiation and measurement. Data are the average of three technical replicates and are representative of three independent experiments. Error bars (within data symbols) are +/- SD.