Figure 5.
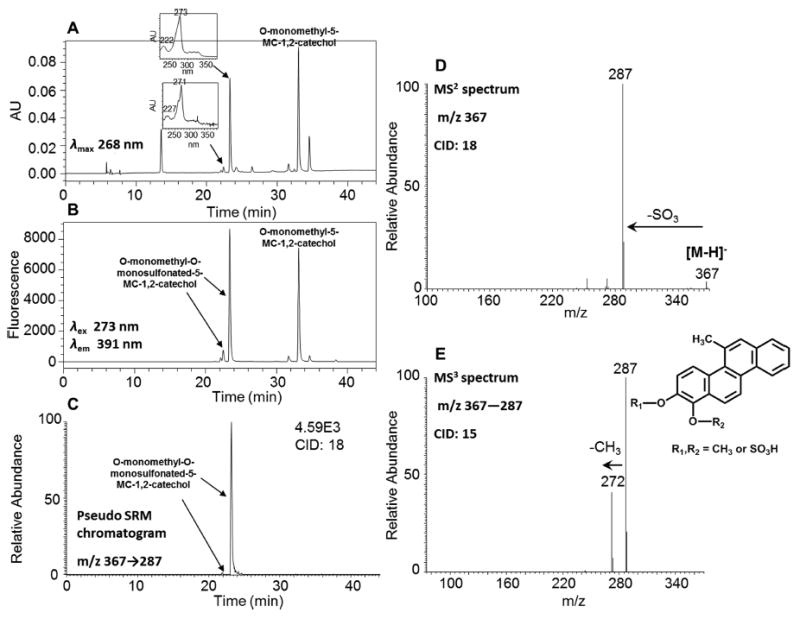
Characterization of synthetic O-monomethyl-O-monosulfonated-5-MC-1,2-catechol. (A) UV chromatogram at λmax 268 nm. (B) FLR chromatogram at λex 273 nm and λem 391 nm. (C) Extracted ion chromatogram of pseudo SRM transition. (D) MS2 spectrum. (E) MS3 spectrum. The product profiles were obtained after 1 h incubation of 5-MC-1,2-catechol with COMT and AdoMet followed by an additional 1 h incubation with SULT1A1 and PAPS.
