Figure 6. Proposed mechanism of cobalt incorporation into the fusion NHase.
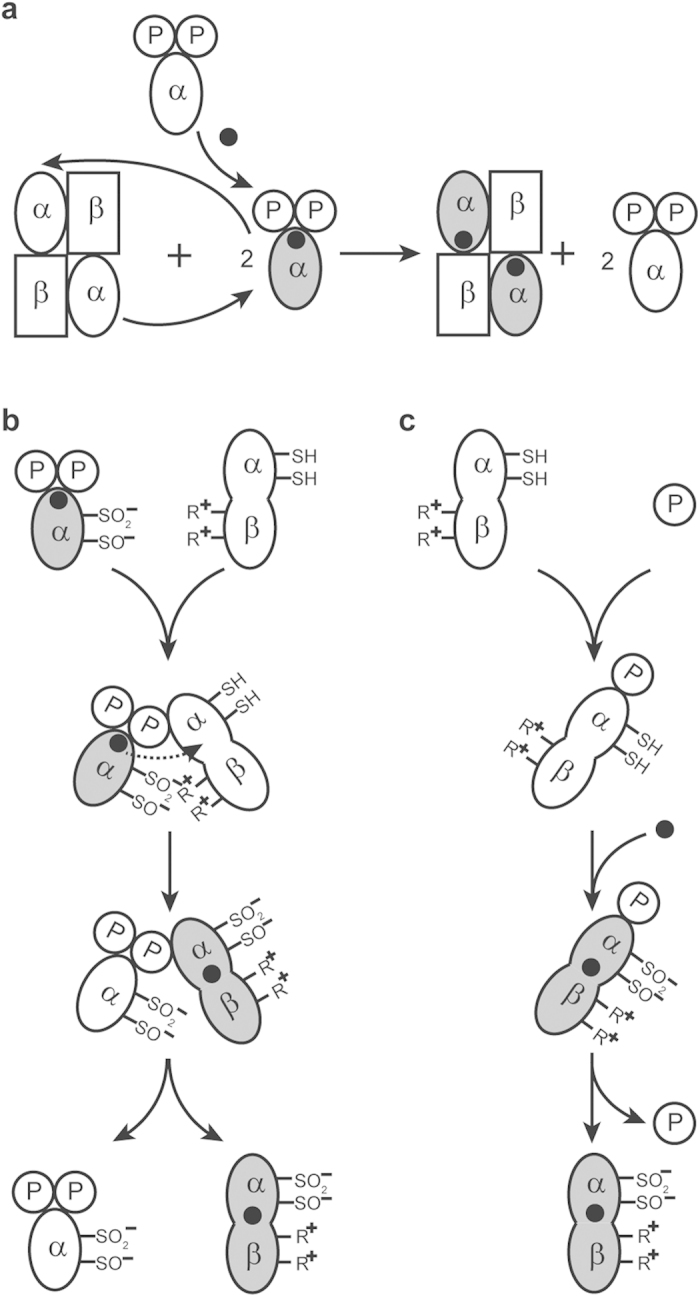
(a) Self-subunit swapping for cobalt incorporation into the wild-type NHase. (b) Cobalt transfer from α(P14K)2 to apo-NHase-(BA)P14K in vitro. The apo α-subunit in apo-NHase-(BA)P14K approaches and binds to the recognition (binding) site of P14K during self-subunit swapping11, and then the β-subunit (of apo-NHase-(BA)P14K) and the cobalt-containing α-subunit (of α(P14K)2) are attracted through the electrostatic interaction and associate with each other to form an intermediate complex. Instead of α-subunit exchange between the two proteins, cobalt ion direct transfer occurs. Oxidation of the two cysteine is responsible for cobalt binding, resulting in active NHase-(BA)P14K. (c) Cobalt incorporation into the fusion apo-NHase-(BA)P14K in vivo. P14K directly contacts with the α-subunit domain of the fusion βα protein because the binding sites in the α-subunit are free due to the fusion, resulting in an intermediate complex for cobalt insertion and the two cysteine oxidation. In these models, one fusion βα protein is used to show apo-NHase-(BA)P14K, the change of the location of the two arginine is used to demonstrate further folding after cobalt incorporation.
