Abstract
Background
Hypoacetylation on histone H3 of human prostate cancer cells has been described. Little is known about the modifications of other histones from prostate cancer cells.
Methods
Histones were isolated from the prostate cancer cell line DU-145 and the non-malignant prostatic cell line RC170N/h. Post-translational modifications of histone H2B were determined by liquid chromatography-mass spectrometry (LC-MS)/MS.
Results
The histone H2B of the prostate cancer cell line DU-145 was found to have hypoacetylation, hypomethylation, and dephosphorylation as compared to the non-malignant prostatic cell line RC170N/h. H2B regained acetylation on multiple lysine residues, phosphorylation on Thr19, and methylation on Lys23 and Lys43 in the DU-145 cells after sodium butyrate treatment.
Conclusions
The histone H2B of DU-145 prostate cancer cells are hypoacetylated, hypomethylated, and dephosphorylated. Histone deacetylase inhibitor reversed this phenotype. Epigenetic agent may therefore be useful for prostate cancer therapy and worth further investigation.
Electronic supplementary material
The online version of this article (doi:10.1186/s13045-016-0233-x) contains supplementary material, which is available to authorized users.
Keywords: Epigenomes, Histone H2B modifications, Mass spectrometry, Acetylation, Deacetylases, Hypoacetylation, Prostate cancer
To the editor
The histone proteins H3 and H4 hetero-tetramer are flanked on each side by an H2A and H2B hetero-dimer, with H3-H4 and H2A-H2B each interacting with different parts of the nucleosomal DNA. Aberrant activities of DNA methyltransferases and acetyltransferases lead to epigenetic remodeling of chromatin and have been implicated in carcinogenesis [1–3]. At this time, not much is known about the post-translational modifications of histones other than H3 in prostate cancer cells. Recent studies in yeast have revealed the importance of H2B in transcriptional regulation [4]. In this study, post-translational modifications of histone H2B from the human prostate cancer cell line DU-145 and the non-malignant prostatic cell line RC170N/h were analyzed by liquid chromatography-mass spectrometry (LC-MS/MS) (see Additional file 1) [5–7]. The status of H2B acetylation in DU-145 cells was illustrated in Fig. 1a, with acetylation determined on a single lysine (K) residue at amino acid sequence position 20 (K20). Lysine at position 23 (K23) was found to be di-methylated as shown in Fig. 1a. The acetylation at K20 and di-methylation at K23 were observed on the tryptic peptides, 16KAVTKAQK23 and 17AVTKAQKKDGKK28.
Fig. 1.

Post-translational modifications on the histone H2B. a The acetylation and methylation sites of H2B in the human prostate cancer DU-145 cells. b The acetylation, methylation, and phosphorylation of H2B in the non-malignant prostatic RC170N/h cells. c Histone H2B modifications in the DU-145 cells after sodium butyrate treatment.  indicates acetylation,
indicates acetylation,  indicates methylation,
indicates methylation,  indicates di-methylation,
indicates di-methylation,  indicates tri-methylation, and
indicates tri-methylation, and  indicates phosphorylation. The H2B histone sequence is presented at the lower part of the figure. The underscored sequences represent the alpha helices in the structured domains of the histone
indicates phosphorylation. The H2B histone sequence is presented at the lower part of the figure. The underscored sequences represent the alpha helices in the structured domains of the histone
Analyses of H2B from RC170N/h cells revealed acetylation at K5, K16, and K20 (Fig. 1b). The methylations were found to be tri-methylated at K15 and K120, di-methylated at K23, and mono-methylated at K116 (Fig. 1b). The same peptides 16KAVTKAQK23 and 17AVTKAQKKDGKK28 that were analyzed for the DU-145 cells, together with other peptides including 1PDPSKSAPAPKKGSKKAVTKAQK23 and 109HAVSEGTKAVTK120, were examined for these modifications. The non-malignant RC170N/h cells clearly had more acetylated and methylated lysine residues on H2B than the DU-145 cancer cells.
To evaluate the histone deacetylase (HDAC) activity, DU-145 cells were treated with sodium butyrate, an inhibitor of HDACs. After butyrate treatment of these cells, acetylation on lysine residues, K5, K11, K12, K16, K20, and K27 of H2B, was identified (Fig. 1c). Specifically, the acetylation changes were detected in the following peptides, 6SAPAPKKGSK15, 16KAVTKAQK23, 1PEPAKSAPAPK11, and 17AVTKAQKKDGKK28. The fact that acetylation on H2B in the DU-145 cells was detected in multiple additional lysine residues after HDAC inhibition by sodium butyrate suggests that there was an excessive HDAC activity in the DU-145 cells. Acetylation of K5, K16, and K20 was also observed in the non-malignant RC170N/h cells (Fig. 1b, c). These data showed that the DU-145 cancer cells had a single K20 acetylation site, compared to the non-malignant RC170N/h cells that had three sites at K5, K16, and K20. The NaB-treated DU-145 cells had six acetylation sites at K5, K11, K12, K16, K20, and K27. The differences in the acetylation sites were detected at the N termini, without involving the alpha helices which start at amino acid residue 37 of H2B (Fig. 1, underscored sequences). The small lung carcinoma cells have six sites at K5, K11, K12, K15, K16, and K20 [8]; The Jurkat cells have three sites at K12, K15, and K20 [9], whereas the untreated DU-145 cells in this study have one acetylated K20. These results indicate that there are clear differences in acetylation sites among the human cell lines. These differences constitute the epigenetic signatures of individual neoplastic clones.
We next examined changes of the H2B methylation status in the DU-145 cells upon HDAC inhibition. After sodium butyrate treatment, additional methylation on K43 was found (Fig. 2c), as compared to only K23 methylation in the untreated DU-145 cells (Fig. 1a).
Fig. 2.
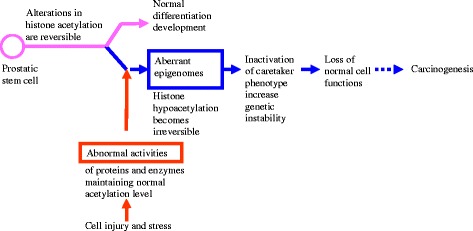
Hypothetical pathways of carcinogenesis from prostatic stem cells. Histone hypoacetylation leads to disruption of the normal epigenome in prostatic stem cells. The aberrant epigenome with hypoacetylation may be established when the reversible alterations in acetylation become irreversible. This is due to the abnormal histone deacetylase activities. As a result, caretaker phenotype and critical genes are inactivated. These eventually lead to carcinogenesis
There was no obvious phosphorylation detected on histone H2B peptides from the DU-145 prostate cancer cells. However, phosphorylation was detected at serines S6 and S14, as well as threonines T19 and T115 of the H2B histone in the non-malignant prostatic RC170N/h cells (Fig. 1b). Phosphorylation was detected on H2B peptides, 16KAVTKAQK23, 1PDPSKSAPAPKKGSKKAVTKAQK23, and 109HAVSEGTKAVTK120. When the DU-145 cells were treated with sodium butyrate (NaB), phosphorylation was found on threonine T19 (Fig. 1c). The increase in phosphorylation of H2B in DU-145 cells after NaB treatment is particularly intriguing. This provides the first evidence of the possible cross talk of HDACs and serine/threonine kinases.
As illustrated in Fig. 2, we postulate that the abnormal HDAC activity could turn the reversible histone modifications into irreversible, leading to perpetual aberrant epigenomes. An enhanced HDAC activity or a reduced K-acetyltransferase activity would tip the balance towards deacetylation. These could perpetuate the epigenetic changes and result in carcinogenesis. Targeting epigenetic modifications by inhibiting HDACs and DNA methyltransferases has become novel cancer therapies [2, 3, 10–12]. This study also suggests that HDAC inhibitors may be a potential therapeutic option for prostate cancer.
In conclusion, the histone H2B of DU-145 prostate cancer cells are hypoacetylated, hypomethylated, and dephosphorylated. Histone deacetylase inhibitor reversed this phenotype. Epigenetic agent may therefore be useful for prostate cancer therapy and worth further investigation.
Abbreviations
- HDAC
histone deacetylase
- K
lysine
- NaB
sodium butyrate
- S
serine
- T
threonine
Additional file
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SC performed all aspects of the experiments. XX evaluated the LC-MS/MS data analysis, interpreted the data, and participated in the manuscript preparation. YM participated in the cell culture preparation. DL and JWC were actively involved in the concept design, study supervision, data analyses, logistic support, and manuscript preparation. All authors read and approved the final manuscript.
Contributor Information
Delong Liu, Email: Delong_liu@nymc.edu.
J. W. Chiao, Email: Jen-Wei_Chiao@nymc.edu
References
- 1.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 2.Tan J, Cang S, Ma Y, Petrillo RL, Liu D. Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. J Hematol Oncol. 2010;3:5. doi: 10.1186/1756-8722-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shenoy N, Vallumsetla N, Zou Y, Galeas J, Shrivastava M, Hu C, et al. Role of DNA methylation in renal cell carcinoma. J Hematol Oncol. 2015;8:88. doi: 10.1186/s13045-015-0180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyrick JJ, Parra MA. The role of histone H2A and H2B post-translational modifications in transcription: a genomic perspective. Biochim Biophys Acta. 1789;2009:37–44. doi: 10.1016/j.bbagrm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Cang S, Feng J, Konno S, Han L, Liu K, Sharma SC, et al. Deficient histone acetylation and excessive deacetylase activity as epigenomic marks of prostate cancer cells. Int J Oncol. 2009;35:1417–22. doi: 10.3892/ijo_00000459. [DOI] [PubMed] [Google Scholar]
- 6.Gu Y, Li H, Miki J, Kim KH, Furusato B, Sesterhenn IA, et al. Phenotypic characterization of telomerase-immortalized primary non-malignant and malignant tumor-derived human prostate epithelial cell lines. Exp Cell Res. 2006;312:831–43. doi: 10.1016/j.yexcr.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Wang LG, Liu XM, Fang Y, Dai W, Chiao FB, Puccio GM, et al. De-repression of the p21 promoter in prostate cancer cells by an isothiocyanate via inhibition of HDACs and c-Myc. Int J Oncol. 2008;33:375–80. [PubMed] [Google Scholar]
- 8.Beck HC, Nielsen EC, Matthiesen R, Jensen LH, Sehested M, Finn P, et al. Quantitative proteomic analysis of post-translational modifications of human histones. Mol Cell Proteomics. 2006;5:1314–25. doi: 10.1074/mcp.M600007-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Bonenfant D, Coulot M, Towbin H, Schindler P, van Oostrum J. Characterization of histone H2A and H2B variants and their post-translational modifications by mass spectrometry. Mol Cell Proteomics. 2006;5:541–52. doi: 10.1074/mcp.M500288-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Hajkova H, Fritz M, Haskovec C, Schwarz J, Salek C, Markova J, et al. CBFB-MYH11 hypomethylation signature and PBX3 differential methylation revealed by targeted bisulfite sequencing in patients with acute myeloid leukemia. J Hematol Oncol. 2014;7:66. doi: 10.1186/s13045-014-0066-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol. 2014;7:11. doi: 10.1186/1756-8722-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han S, Kim Y-J, Lee J, Jeon S, Hong T, Park G-j, et al. Model-based adaptive phase I trial design of post-transplant decitabine maintenance in myelodysplastic syndrome. J Hematol Oncol. 2015;8:118. doi: 10.1186/s13045-015-0208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Zhou J, Miki J, Furusato B, Gu Y, Srivastava S, et al. Telomerase-immortalized non-malignant human prostate epithelial cells retain the properties of multipotent stem cells. Exp Cell Res. 2008;314:92–102. doi: 10.1016/j.yexcr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Wang LG, Beklemisheva A, Liu XM, Ferrari AC, Feng J, Chiao JW. Dual action on promoter demethylation and chromatin by an isothiocyanate restored GSTP1 silenced in prostate cancer. Mol Carcinog. 2007;46:24–31. doi: 10.1002/mc.20258. [DOI] [PubMed] [Google Scholar]
- 15.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–6. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 16.Rauen KA, Sudilovsky D, Le JL, Chew KL, Hann B, Weinberg V, et al. Expression of the coxsackie adenovirus receptor in normal prostate and in primary and metastatic prostate carcinoma: potential relevance to gene therapy. Cancer Res. 2002;62:3812–8. [PubMed] [Google Scholar]


