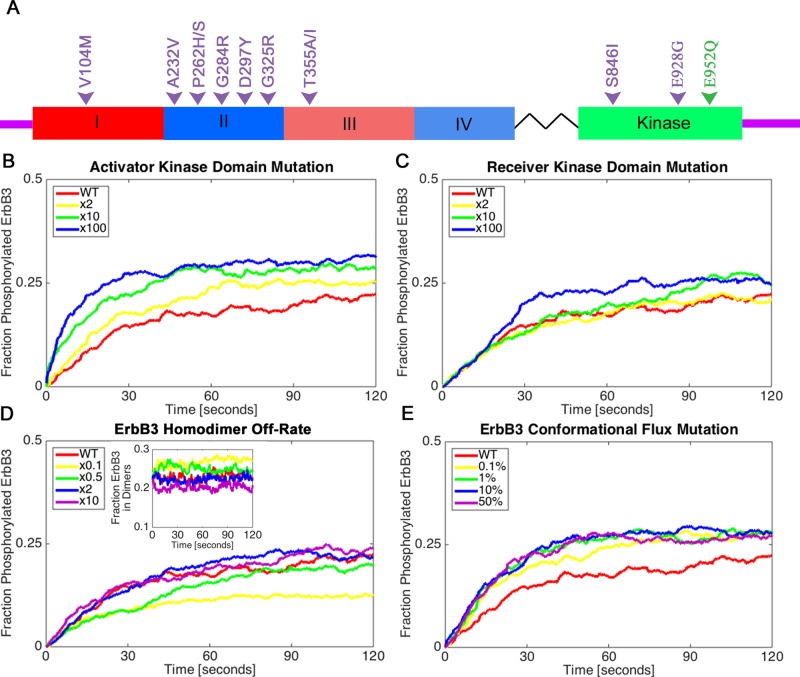
FIGURE 6:
Classes of ErbB3 oncogenic mutations can differentially affect receptor phosphorylation. (A) Diagram of the ErbB3 receptor with hot spot mutations from Jaiswal et al. (2013; purple), as well as the E933Q mutation from this study (E952Q based on the alternative numbering system, green). Hot spot mutations occur in different ErbB3 domains and are potentially linked to distinct mechanistic classes. (B–E) Four possible mechanistic classes explored by varying multipliers within the simulation. All simulations were initiated with 50% ligand-bound ErbB3. The fraction of ErbB3 receptors phosphorylated over time is shown for each class of mutations. Shown are the effects of (B) an ErbB3 kinase domain mutation that causes the activator receptor to more efficiently activate its partner kinase, (C) a mutation in the kinase domain that increases kinase activity in the receiver, (D) mutations that increase or decrease the dimer off-rate (inset shows the fraction of ErbB3 receptors in dimers), and (E) a mutation that increases the stability of the extended, active conformation of ErbB3 independent of ligand binding. In E, the reported range is 0.1–50% of unliganded ErbB3 receptors in the extended conformation, by comparison to 0.01% as the original flux rate for wild-type receptors.