Abstract
Vertical transmission may contribute to the maintenance of arthropod-borne viruses, but its existence in chikungunya virus (CHIKV) is unclear. Experimental vertical transmission of infectious clones of CHIKV in Aedes aegypti mosquitoes from Malaysia was investigated. Eggs and adult progeny from the second gonotrophic cycles of infected parental mosquitoes were tested. Using polymerase chain reaction (PCR), 56.3% of pooled eggs and 10% of adult progeny had detectable CHIKV RNA, but no samples had detectable infectious virus by plaque assay. Transfected CHIKV RNA from PCR-positive eggs did not yield infectious virus in BHK-21 cells. Thus, vertical transmission of viable CHIKV was not demonstrated. Noninfectious CHIKV RNA persists in eggs and progeny of infected Ae. aegypti, but the mechanism and significance are unknown. There is insufficient evidence to conclude that vertical transmission exists in CHIKV, as positive results reported in previous studies were almost exclusively based only on viral RNA detection.
Introduction
Chikungunya virus (CHIKV) is an arthropod-borne alphavirus belonging to the Togaviridae family, which is transmitted to humans mainly by Aedes aegypti and Aedes albopictus. In Asia, Ae. aegypti mosquitoes are the primary vector in an urban cycle of transmission. CHIKV causes acute fever, myalgia, rash, and arthralgia, and the latter may persist for years.1 There are three main CHIKV genotypes: West African, East/Central/South African (ECSA), and Asian. Recent strains of the ECSA genotype form two sublineages, Indian and Indian Ocean.2 Over the past 10 years, CHIKV has reemerged to cause worldwide epidemics affecting millions. These include epidemic ECSA strains spreading from East Africa to the islands and land masses adjacent to the Indian Ocean and Asia (Indian sublineage), including Malaysia,2 and the Asian genotype spreading to the Americas.1
Vertical transmission of arboviruses occurs from an adult female mosquito to its eggs and progeny. This may be an important source of maintenance for arboviruses such as dengue virus3–5 and West Nile virus,6,7 particularly during unfavorable environmental conditions. Inconsistent findings are reported for the existence of vertical transmission of CHIKV; if it does occur, it is infrequent.8,9 The aim of this study was to determine the occurrence of experimental vertical transmission using CHIKV from each ECSA sublineage (Indian and Indian Ocean) in Ae. aegypti mosquitoes from Malaysia. CHIKV undergoes local adaptation to mosquitoes in different geographical settings, leading to increased midgut replication and dissemination, although effects on vertical transmission have not been specifically studied.10 If vertical transmission is shown to occur in Malaysian mosquitoes, we examined whether it would occur to a greater extent in CHIKV of the Indian sublineage, which caused large outbreaks in Malaysia, rather than CHIKV of the Indian Ocean sublineage, which have not been reported in Malaysia.
Materials and Methods
Infectious clones.
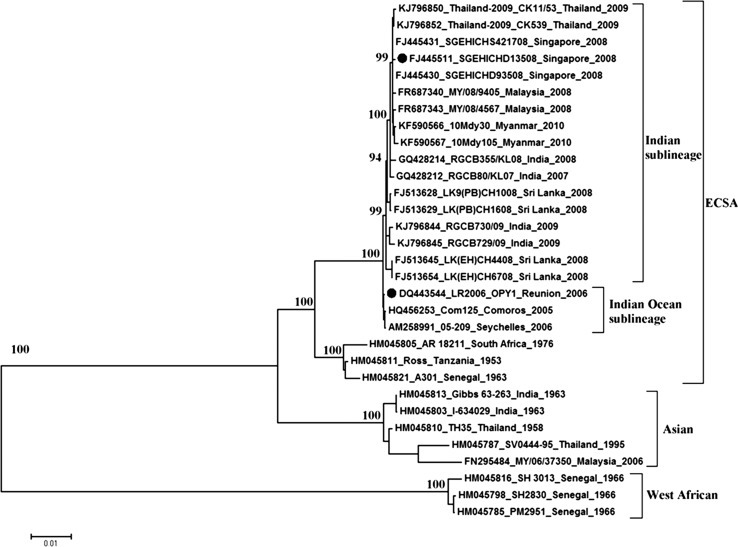
Full-length infectious cDNA clones were constructed as previously described11 for the two CHIKV strains used: ICRES1 (based on LR2006_OPY1, a virus of Indian Ocean sublineage, isolated in Reunion Island; GenBank accession number: DQ443544) and SGP011 (based on SGEHICHD13508, a virus of the Indian sublineage isolated in Singapore; FJ445511),12 which is closely related to Malaysian viruses (Figure 1 ).
Figure 1.
Phylogenetic analysis of full coding sequences of chikungunya virus (CHIKV). A maximum likelihood tree was drawn with MEGA5, using the general time reversible model with proportion of invariant sites and gamma-distributed rates among sites (GTR + I + G), and inferred following bootstrap analysis with 1,000 replicates. The scale shows branch lengths measured in the number of substitutions per site. The three main genotypes are shown: West African, Asian, and East/Central/South African (ECSA). The infectious clones ICRES1 and SGP011 used in this study were based on the sequences with accession numbers DQ443544 and FJ445511, respectively, and are indicated by (•).
Viruses were rescued by electroporation of in vitro transcribed infectious transcripts from corresponding cDNA clones in BHK-21 cells (ATCC no., CCL-10). Plasmids were first linearized with Not I restriction endonuclease, followed by in vitro transcription from the minimal SP6 promoter as described previously.13 Approximately 10 μg of in vitro transcribed RNA were electroporated into 106 BHK-21 cells using an electroporator with a 4 mm gap electroporation cuvette. Cells were pulsed twice with a square wave protocol, with a 240V pulse for 3 seconds and a time constant after each pulse of 25 milliseconds. Cell culture supernatants were harvested 24 hours postelectroporation and virus titers in Ae. aegypti were measured by plaque assay.13 Mosquitoes were fed with infectious blood meal containing either of the CHIKV strains used, and 10 individual mosquitoes were collected at each time point of 0, 1, 2, 3, 5, 7, and 10 days postinfection (dpi).
Preparation of homogenate from mosquitoes and eggs.
To determine replication kinetics of infectious clones, midguts were dissected out using clean needles which were soaked in 70% alcohol between each mosquito. To determine the presence of infectious virus in parental and adult progeny mosquitoes, whole mosquitoes were homogenized. Mosquito eggs were homogenized in pools from an individual parent mosquito. All samples were homogenized in tubes containing zirconium beads and 500 μL of serum-free medium.
Plaque assay.
The virus titers of mosquito and egg homogenates were quantified using plaque assay. Samples were serially diluted, and 400 μL of each dilution were added to duplicate wells of six-well plates containing 80–90% confluent monolayers of BHK-21 cells, supplemented with growth medium containing carboxymethylcellulose sodium salt. Plaque forming units (pfu) were counted after staining with crystal violet. Virus titers are reported as pfu/mL of homogenate.
Vertical transmission of CHIKV in mosquitoes.
Laboratory-reared Ae. aegypti (Seputeh strain, collected in Kuala Lumpur, Malaysia) were used. Female mosquitoes aged 3–6 days were fed with 6.1 × 105 pfu/mL of each CHIKV strain in blood donated by Wan Yusof Wan Sulaiman, who is seronegative for CHIKV antibodies.2 Control mosquitoes were fed uninfected blood. The mosquitoes were provided the blood meal using a Hemotek feeder (Hemotek Ltd., United Kingdom) at 37°C for 1 hour in the dark. Fully engorged mosquitoes were maintained at 28 ± 1°C with 88% relative humidity and a 12 hour: 12 hour photoperiod. Mosquitoes were fed with 10% sucrose supplemented with vitamin B complex until 7 dpi, then starved for 24 hours before being fed a clean blood meal. Fully engorged mosquitoes were individually separated and set for oviposition, and eggs from the second gonotrophic cycle were collected at 11–13 dpi. Parental adult homogenates were tested with reverse transcription-polymerase chain reaction (RT-PCR) targeting the region encoding for E1 protein of CHIKV, as well as plaque assay to detect infectious virus. All PCR-positive homogenates prepared from parental adults were confirmed to have infectious virus titers of at least 104 pfu/mL. Eggs from PCR-positive and plaque assay-positive parental adults were further processed, with half being homogenized for RT-PCR (regions encoding E1 and the nonstructural proteins nsP1 and nsP3) and plaque assay, while the other half were reared under standard laboratory conditions until adulthood. Progeny adult homogenates were also analyzed by RT-PCR targeting the region encoding E1 and plaque assay. The eggs from the first gonotrophic cycle were not examined as vertical transmission is more likely to occur in second and subsequent cycles following infection with CHIKV,14 probably due to a longer extrinsic incubation period allowing replication in the ovaries.
Reverse transcription-polymerase chain reaction.
Previously published RT-PCR protocols were used for the detection of regions encoding E1,15 nsP1,15 and nsP316 of CHIKV. The identities of E1, nsP1, and nsP3 region-specific products were all confirmed by sequencing.
Transfection of CHIKV RNA.
To test if CHIKV RNA detected in eggs is infectious, CHIKV RNA was extracted from the egg homogenates and transfected into BHK-21 cells using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA) according to manufacturer's protocols. Two different positive controls were used, which successfully yielded infectious virus: CHIKV RNA alone, and, to exclude inhibitory effects from mosquito RNA, CHIKV RNA mixed with extracted total RNA from mosquito eggs which were PCR-negative for CHIKV. Cell supernatants were collected at 2 dpi and tested for CHIKV RNA and infectious virus.
Results and Discussion
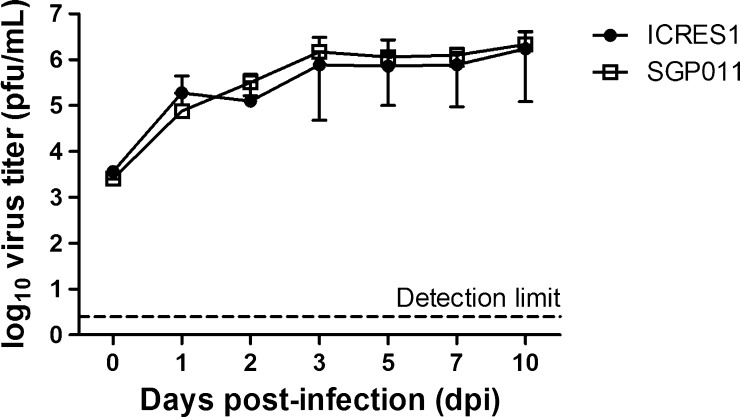
Both infectious clones replicated stably and similarly in the midguts of Ae. aegypti (Figure 2 ). Both ICRES1 and SGP011 reached similar peak titers of 1.7 × 106 pfu/mL and 2.1 × 106 pfu/mL, respectively, at 10 dpi.
Figure 2.
Replication kinetics of two different strains of chikungunya virus (CHIKV) infectious clones in the midguts of Aedes aegypti. Virus titers were measured by plaque assay in BHK-21 cells and refer to plaque forming units per milliliter of homogenate. Error bars represent standard deviations of means of positive titers from 4 to 10 infected individual mosquitoes at each time point.
A total of 32 adult females that laid eggs were confirmed to be positive by RT-PCR. Of these, 11 were infected with ICRES1 and 21 were infected with SGP011 (Table 1). All control mosquitoes tested negative by RT-PCR. CHIKV RNA was detected from 18/32 (56.3%) of the total pools of eggs from the second gonotrophic cycle, and 5/50 (10%) of the adult mosquitoes reared from these eggs. There were no significant differences in RNA detection rates between the two virus strains used. However, although CHIKV RNA was detectable in the eggs and progeny of infected Ae. aegypti, none was found to contain infectious virus detectable by plaque assay. The homogenate prepared from progeny adults were further passaged another three times in BHK-21 cells, after which neither infectious virus nor RNA was detectable. CHIKV RNA extracted from the eggs homogenate was also transfected into BHK-21 cells, to see if infectious virus could be generated. The transfected CHIKV RNA from the eggs homogenate produced no infectious virus, and CHIKV RNA was no longer detectable in the transfected BHK-21 after two further passages. This suggests that the CHIKV RNA detected in the eggs homogenate was noninfectious.
Table 1.
Detection rates of CHIKV RNA and infectious virus in Aedes aegypti eggs and progeny
| CHIKV ICRES1 blood-fed parental mosquitoes (N = 11) | CHIKV SGP011 blood-fed parental mosquitoes (N = 21) | |||
|---|---|---|---|---|
| RNA | Infectious virus | RNA | Infectious virus | |
| Eggs (pools) | 5/11 (45.5%) | 0 | 13/21 (61.9%) | 0 |
| Progeny adult mosquitoes | 4/22 (18.2%) | 0 | 1/28 (3.6%) | 0 |
CHIKV = chikungunya virus.
For comparison, previous studies of vertical transmission of CHIKV in Aedes mosquitoes were summarized (Table 2). RT-PCR was the most common method used to detect CHIKV, in seven of nine studies,8,9,14,18,20–22 and only one of those failed to detect CHIKV RNA.22 RNA detection rates otherwise ranged between 0.4% and 100%, in both experimentally and naturally infected samples. Notably, only five studies used methods to detect either viral protein expression (immunofluorescence staining17,22) or viable infectious virus (cell culture9,20 and in vivo inoculation19). Only one of these studies demonstrated the presence of infectious virus, in Ae. albopictus adults reared from larvae from outbreak sites; this required three passages of obtained materials in Vero cells, and ultimately yielded unusually high levels of 10 pfu/mL in cell culture supernatants.20 Thus, the inconsistent results reported for vertical transmission in CHIKV appear to be mainly due to the assay used to detect the virus: viral RNA has been detected by many researchers, while infectious virus has not.
Table 2.
Summary of previous studies of vertical transmission of chikungunya virus (CHIKV) in Aedes mosquitoes
| Location | Species | Mosquitoes tested | Assays used | Reference | ||
|---|---|---|---|---|---|---|
| RT-PCR | IF | Virus culture | ||||
| India | Aedes aegypti | Larvae and adult progeny of experimentally infected mosquitoes | Ae. aegypti: 8/30 larvae (26.6%) and 14/33 adults (42.4%) | Not done | Not done | 14 |
| India | Ae. aegypti, Aedes albopictus | Adult progeny of experimentally infected mosquitoes (three gonotrophic cycles) | Not done | Ae. aegypti: 0/3,814 (0%); Ae. albopictus: 0/1,754 (0%) | Not done | 17 |
| Thailand | Ae. aegypti, Ae. albopictus | Male adults collected from affected sites | Ae. aegypti: 6/28 pools (21.4%); Ae. albopictus: 2/2 pools (100%) | Not done | Not done | 18 |
| South Africa | Ae. aegypti, Ae. furcifer | Adult progeny of experimentally infected mosquitoes | Not done | Not done | In mice. Ae. aegypti: 0/3,158 adults (0%); Ae. furcifer: 0/1,395 adults (0%) | 19 |
| India | Ae. albopictus | Adults reared from larvae from affected sites | Ae. albopictus: 3/3 pools (100%) | Not done | Virus detected, after three passages in Vero cells | 20 |
| Reunion Island | Ae. albopictus | Adults reared from larvae from affected sites | Ae. albopictus: 2/502 adults (0.4%) | Not done | Not done | 21 |
| Reunion Island | Ae. albopictus | Adult progeny of experimentally infected mosquitoes (G1 and G2) | G1: 0/1,675 (0%); G2: 0/1,709 (0%) | Not done | 22 | |
| Italy | Ae. albopictus | Adult progeny of experimentally infected mosquitoes (three gonotrophic cycles) | Ae. albopictus: 3/689 adults (0.4%) | Not done | Not done | 8 |
| Madagascar | Ae. albopictus | Adults reared from larvae from affected sites | Ae. albopictus: 5/23 pools (21.8%) | Not done | No growth in AP61 cells | 9 |
CHIKV = chikungunya virus; G1 = first gonotrophic cycle; G2 = second gonotrophic cycle; IF = immunofluorescence assay; RT-PCR = reverse transcription-polymerase chain reaction.
Definitive demonstration of successful vertical transmission of infectious CHIKV is important, as this is a significant gap in the understanding of CHIKV maintenance outside the human host. Other alphaviruses have shown evidence of viable vertical transmission in mosquitoes and other insects. Examples of these include: western equine encephalitis virus, isolated from adult Aedes dorsalis collected as larvae23; Ndumu virus, cultured from Culex pipiens grown from field-collected larvae24; and Buggy Creek virus, isolated from eggs of Oeciacus vicarius swallow bugs.25
On the basis of the findings of this and other studies, it should be concluded that detection of viral RNA itself is insufficient to confirm the occurrence of vertical transmission of CHIKV. It is possible that the detection of only viral RNA may be due to the relative insensitivity of the plaque assay technique used, which had a limit of detection of 2.5 pfu/mL, while the limit of detection of the RT-PCR assay used to detect the region encoding E1 was as low as 0.08 pg of CHIKV RNA. Alternatively, viral RNA may represent inactivated virus, which may remain detectable even in dead mosquitoes stored for many weeks at 28°C.26 The desiccation-resistant nature of Aedes eggs may enhance the stability of viral nucleic acid. Viral RNA may also represent a defective or latent form of persistent virus. In our study, the failure to produce infectious virus following transfection of CHIKV RNA obtained from the egg homogenates may be because the detected CHIKV RNA is incomplete, defective, or possibly in the form of a double-stranded replication intermediate, all of which would not allow translation of the nonstructural proteins required to start the infection cycle. Long-term persistence of the flavivirus West Nile virus RNA, but not live virus, has been reported in avian hosts up to 36 weeks postinfection.27 However, it is not known if the virus can recrudesce to render the bird infectious to arthropods once more. Similarly, bluetongue virus RNA but not infectious virus was detected in Culicoides midge larvae from the field.28 Segments of the viral RNA encoding the outer capsid genes, required for growth in insect cells, were downregulated; it was hypothesized that this allows bluetongue virus to survive as a persistent, noncytopathic infection of the insect host which is inactive over winter, but with the ability to reactivate later. Another alphavirus, Sindbis virus, has been shown to persist in a nonproductive RNA form in immunodeficient mouse brains, and can be reactivated as infectious virus after several months.29 It has been recently reported that Sindbis virus may replicate as noncapped genomes with extremely reduced yield of infectious virus.30 Therefore, the possibility exists that CHIKV RNA is present in a latent form in eggs or progeny before an unknown reactivating stimulus. In general, our findings are analogous with other studies which have been unable to isolate infectious virus from mammals with persistently detectable CHIKV RNA.31,32
In conclusion, CHIKV RNA persists for prolonged periods in mosquito eggs and is also detectable in hatched adults. However, in the absence of detection of infectious virus particles, this is not definitive evidence of vertical transmission, which remains largely unproven for CHIKV. Future research should explore the potential significance of persistent viral RNA in eggs and progeny, and standardize the detection assay to confirm vertical transmission.
ACKNOWLEDGMENTS
We thank Lisa F. P. Ng of the Singapore Immunology Network, Agency for Science, Technology (A*STAR), Singapore for providing the SGP011 infectious clone.
Footnotes
Financial support: This study was funded by the European Union's Seventh Framework Program (Integrated Chikungunya Research, grant agreement no. 261202), the Ministry of Higher Education, Malaysia (FRGS grant FP036-2013A), and University of Malaya (PPP grant PG085-2014B and HIR grant E000013-20001).
Authors' addresses: Hui Vern Wong, Yoke Fun Chan, and I-Ching Sam, Department of Medical Microbiology, Faculty of Medicine, University Malaya, Kuala Lumpur, Malaysia, E-mails: whvern@gmail.com, chanyf@ummc.edu.my, and jicsam@ummc.edu.my. Indra Vythilingam and Wan Yusof Wan Sulaiman, Department of Parasitology, Faculty of Medicine, University Malaya, Kuala Lumpur, Malaysia, E-mails: indrav@um.edu.my and wanyus@um.edu.my. Aleksei Lulla and Andres Merits, Institute of Technology, University of Tartu, Estonia, E-mails: aleksei.lulla@ut.ee and andres.merits@ut.ee.
References
- 1.Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372:1231–1239. doi: 10.1056/NEJMra1406035. [DOI] [PubMed] [Google Scholar]
- 2.Sam IC, Loong SK, Michael JC, Chua CL, Wan Sulaiman WY, Vythilingam I, Chan SY, Chiam CW, Yeong YS, AbuBakar S, Chan YF. Genotypic and phenotypic characterization of chikungunya virus of different genotypes from Malaysia. PLoS One. 2012;7:e50476. doi: 10.1371/journal.pone.0050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohani A, Aidil Azahary AR, Malinda M, Zurainee MN, Rozilawati H, Wan Najdah WM, Lee HL. Eco-virological survey of Aedes mosquito larvae in selected dengue outbreak areas in Malaysia. J Vector Borne Dis. 2014;51:327–332. [PubMed] [Google Scholar]
- 4.Martins VE, Alencar CH, Kamimura MT, de Carvalho Araujo FM, De Simone SG, Dutra RF, Guedes MI. Occurrence of natural vertical transmission of dengue-2 and dengue-3 in Aedes aegypti and Aedes albopictus in Fortaleza, Ceara, Brazil. PLoS One. 2012;7:e41386. doi: 10.1371/journal.pone.0041386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckner EA, Alto BW, Lounibos LP. Vertical transmission of Key West dengue-1 virus by Aedes aegypti and Aedes albopictus (Diptera: Culicidae) mosquitoes from Florida. J Med Entomol. 2013;50:1291–1297. doi: 10.1603/me13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra AC, Mourya DT. Transovarial transmission of West Nile virus in Culex vishnui mosquito. Indian J Med Res. 2001;114:212–214. [PubMed] [Google Scholar]
- 7.Nelms BM, Fechter-Leggett E, Carroll BD, Macedo P, Kluh S, Reisen WK. Experimental and natural vertical transmission of West Nile virus by California Culex (Diptera: Culicidae) mosquitoes. J Med Entomol. 2013;50:371–378. doi: 10.1603/me12264. [DOI] [PubMed] [Google Scholar]
- 8.Bellini R, Medici A, Calzolari M, Bonilauri P, Cavrini F, Sambri V, Angelini P, Dottori M. Impact of chikungunya virus on Aedes albopictus females and possibility of vertical transmission using the actors of the 2007 outbreak in Italy. PLoS One. 2012;7:e28360. doi: 10.1371/journal.pone.0028360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratsitorahina M, Harisoa J, Ratovonjato J, Biacabe S, Reynes JM, Zeller H, Raoelina Y, Talarmin A, Richard V, Soares JL. Outbreak of dengue and chikungunya fevers, Toamasina, Madagascar, 2006. Emerg Infect Dis. 2008;14:1135–1137. doi: 10.3201/eid1407.071521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsetsarkin KA, Chen R, Yun R, Rossi SL, Plante KS, Guerbois M, Forrester N, Perng GC, Sreekumar E, Leal G, Huang J, Mukhopadhyay S, Weaver SC. Multi-peaked adaptive landscape for chikungunya virus evolution predicts continued fitness optimization in Aedes albopictus mosquitoes. Nat Commun. 2014;5:4084. doi: 10.1038/ncomms5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pohjala L, Utt A, Varjak M, Lulla A, Merits A, Ahola T, Tammela P. Inhibitors of alphavirus entry and replication identified with a stable chikungunya replicon cell line and virus-based assays. PLoS One. 2011;6:e28923. doi: 10.1371/journal.pone.0028923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Her Z, Malleret B, Chan M, Ong EK, Wong SC, Kwek DJ, Tolou H, Lin RT, Tambyah PA, Rénia L, Ng LF. Active infection of human blood monocytes by chikungunya virus triggers an innate immune response. J Immunol. 2010;184:5903–5913. doi: 10.4049/jimmunol.0904181. [DOI] [PubMed] [Google Scholar]
- 13.Tsetsarkin K, Higgs S, Mcgee CE, De Lamballerie X, Charrel RN, Vanlandingham DL. Infectious clones of chikungunya virus (La Reunion isolate) for vector competence studies. Vector Borne Zoonotic Dis. 2006;6:325–337. doi: 10.1089/vbz.2006.6.325. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal A, Dash PK, Singh AK, Sharma S, Gopalan N, Rao PV, Parida MM, Reiter P. Evidence of experimental vertical transmission of emerging novel ECSA genotype of chikungunya virus in Aedes aegypti. PLoS Negl Trop Dis. 2014;8:e2990. doi: 10.1371/journal.pntd.0002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasebe F, Parquet MC, Pandey BD, Mathenge EG, Morita K, Balasubramaniam V, Saat Z, Yusop A, Sinniah M, Natkunam S, Igarashi A. Combined detection and genotyping of chikungunya virus by a specific reverse transcription-polymerase chain reaction. J Med Virol. 2002;67:370–374. doi: 10.1002/jmv.10085. [DOI] [PubMed] [Google Scholar]
- 16.Chiam CW, Chan YF, Loong SK, Yong SS, Hooi PS, Sam IC. Real-time polymerase chain reaction for diagnosis and quantitation of negative strand of chikungunya virus. Diagn Microbiol Infect Dis. 2013;77:133–137. doi: 10.1016/j.diagmicrobio.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 17.Mourya DT. Absence of transovarial transmission of chikungunya virus in Aedes aegypti and Ae. albopictus mosquitoes. Indian J Med Res. 1987;85:593–595. [PubMed] [Google Scholar]
- 18.Thavara U, Tawatsin A, Pangsakul T, Bhakdeenuan P, Chanama S, Anantapreecha S, Molito C, Chompoosri J, Thammapalo S, Sawanpanyalert P, Siriyasatien P. Outbreak of chikungunya fever in Thailand and virus detection in field population of vector mosquitoes, Aedes aegypti (L.) and Aedes albopictus Skuse (Diptera: Culicidae) Southeast Asian J Trop Med Public Health. 2009;40:951–962. [PubMed] [Google Scholar]
- 19.Jupp PG, McIntosh BM, Santos ID, DeMoor P. Laboratory vector studies on six mosquito and one tick species with chikungunya virus. Trans R Soc Trop Med Hyg. 1981;75:15–19. doi: 10.1016/0035-9203(81)90005-5. [DOI] [PubMed] [Google Scholar]
- 20.Niyas KP, Abraham R, Unnikrishnan RN, Mathew T, Nair S, Manakkadan A, Issac A, Sreekumar E. Molecular characterization of chikungunya virus isolates from clinical samples and adult Aedes albopictus mosquitoes emerged from larvae from Kerala, south India. Virol J. 2010;7:189. doi: 10.1186/1743-422X-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delatte H, Paupy C, Dehecq JS, Thiria J, Failloux AB, Fontenille D. Aedes albopictus, vector of chikungunya and dengue viruses in Reunion Island: biology and control. Parasite. 2008;15:3–13. doi: 10.1051/parasite/2008151003. [DOI] [PubMed] [Google Scholar]
- 22.Vazeille M, Mousson L, Failloux AB. Failure to demonstrate experimental vertical transmission of the epidemic strain of chikungunya virus in Aedes albopictus from La Réunion Island, Indian Ocean. Mem Inst Oswaldo Cruz. 2009;104:632–635. doi: 10.1590/s0074-02762009000400017. [DOI] [PubMed] [Google Scholar]
- 23.Fulhorst CF, Hardy JL, Eldridge BF, Presser SB, Reeves WC. Natural vertical transmission of western equine encephalomyelitis virus in mosquitoes. Science. 1994;263:676–678. doi: 10.1126/science.8303276. [DOI] [PubMed] [Google Scholar]
- 24.Lutomiah J, Ongus J, Linthicum KJ, Sang R. Natural vertical transmission of Ndumu virus in Culex pipiens (Diptera: Culicidae) mosquitoes collected as larvae. J Med Entomol. 2014;51:1091–1095. doi: 10.1603/me14064. [DOI] [PubMed] [Google Scholar]
- 25.Brown C, Moore AT, Young GR, Padhi A, Komar N. Isolation of buggy creek virus (Togaviridae: Alphavirus) from field-collected eggs of Oeciacus vicarius (Hemiptera: Cimicidae) J Med Entomol. 2009;46:375–379. doi: 10.1603/033.046.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mavale M, Sudeep A, Gokhale M, Hundekar S, Parashar D, Ghodke Y, Arankalle V, Mishra AC. Persistence of viral RNA in chikungunya virus-infected Aedes aegypti (Diptera: Culicidae) mosquitoes after prolonged storage at 28°C. Am J Trop Med Hyg. 2012;86:178–180. doi: 10.4269/ajtmh.2012.11-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wheeler SS, Langevin SA, Brault AC, Woods L, Carroll BD, Reisen WK. Detection of persistent West Nile virus RNA in experimentally and naturally infected avian hosts. Am J Trop Med Hyg. 2012;87:559–564. doi: 10.4269/ajtmh.2012.11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White DM, Wilson WC, Blair CD, Beaty BJ. Studies on overwintering of bluetongue viruses in insects. J Gen Virol. 2005;86:453–462. doi: 10.1099/vir.0.80290-0. [DOI] [PubMed] [Google Scholar]
- 29.Levine B, Griffin DE. Persistence of viral RNA in mouse brains after recovery from acute alphavirus encephalitis. J Virol. 1992;66:6429–6435. doi: 10.1128/jvi.66.11.6429-6435.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sokoloski KJ, Haist KC, Morrison TE, Mukhopadhyay S, Hardy RW. Noncapped alphavirus genomic RNAs and their role during infection. J Virol. 2015;89:6080–6092. doi: 10.1128/JVI.00553-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, Denizot M, Guichard E, Ribera A, Henni T, Tallet F, Moiton MP, Gauzère BA, Bruniquet S, Jaffar Bandjee Z, Morbidelli P, Martigny G, Jolivet M, Gay F, Grandadam M, Tolou H, Vieillard V, Debré P, Autran B, Gasque P. Persistent chronic inflammation and infection by chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184:5914–5927. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 32.Poo YS, Rudd PA, Gardner J, Wilson JA, Larcher T, Colle MA, Le TT, Nakaya HI, Warrilow D, Allcock R, Bielefeldt-Ohmann H, Schroder WA, Khromykh AA, Lopez JA, Suhrbier A. Multiple immune factors are involved in controlling acute and chronic chikungunya virus infection. PLoS Negl Trop Dis. 2014;8:e3354. doi: 10.1371/journal.pntd.0003354. [DOI] [PMC free article] [PubMed] [Google Scholar]