Abstract
Hepatitis E virus (HEV) infection is severe during pregnancy, with a pregnant case fatality rate around 30%. In Bangladesh, plasma samples from 1,100 women during the first trimester (TM) and third TM of pregnancy and 3 months postpartum (PP) were tested for anti-HEV IgG. During this time, 40 women developed antibody responses to HEV. These seroconverters are classified as the cases (incidence = 46 infections per 1,000 person-years). All except one seroconversion occurred between the third TM and 3 months PP. The cases and 40 matched non-seroconverters (controls) underwent analysis of a panel of 10 cytokines, 12 vitamins and minerals, and two markers of inflammation. Throughout pregnancy, seroconverting cases displayed higher concentrations of both pro- and anti-inflammatory cytokines compared with the non-seroconverting controls, even prior to infection. In the first TM, seroconverters had lower circulating zinc concentrations (P = 0.03), an increased prevalence of vitamin D deficiency (25-hydroxy vitamin D [25(OH)2D] < 50 nmol/L, P = 0.08), and anemia (hemoglobin < 110 g/L, P = 0.05) compared with controls. There were no differences in C-reactive protein or α-1-acid glycoprotein. Antecedent micronutrient deficiencies may lead to dysregulated cytokine expression and immunologic compromise, increasing the risk of HEV infection, especially during pregnancy. This exploratory analysis reveals potential novel associations that deserve further study.
Introduction
Hepatitis E virus (HEV) is a leading cause of acute viral hepatitis globally, causing an estimated 20.1 million infections every year.1 Large outbreaks, affecting hundreds or thousands of people, have been documented throughout south Asia and Africa.2,3 Although large outbreaks have not been documented in Europe or the United States, autochthonous cases of HEV have been increasingly recognized in the past several years.4,5
HEV typically causes an acute, self-limiting illness similar in clinical presentation to hepatitis A, with about a 3% case fatality rate in the general population.6 However, during pregnancy, HEV infection can lead to fulminant hepatic failure, membrane rupture, spontaneous abortions, and stillbirths.7 Pregnant women infected with HEV experience a case fatality rate of about 30%, a finding confirmed in multiple settings.7 In Bangladesh, nearly 10% of maternal deaths have been attributed to hepatitis, likely an infection with HEV, with a similarly elevated proportion of neonatal deaths caused by this virus.8,9 The exact mechanism of this increased morbidity and mortality during pregnancy is unknown. It remains unclear whether immunologic changes in pregnancy result in increased risk of infection and inadequate control of the infection compared with the general population or whether the T-helper cell (Th) type 2–biased state of the immune system during late pregnancy leads to an immunopathologic response to HEV, fulminant hepatic failure, and death. Furthermore, inconsistent observations of maternal mortality across populations add another layer of complexity to our understanding of this phenomenon. In Egypt, for example, very low levels of maternal mortality subsequent to HEV infections have been observed, despite an identical HEV genotype as seen in south Asia.10 The range of outcomes of infection, from transient infection to severe disease, with the same genotype of HEV likely reflects complex interactions between the host, virus, and environment. Over the past several decades, our group and others have conducted large population-based epidemiologic studies, specifically in cohorts of pregnant women, where this spectrum of outcomes has also been documented.11–13 On the basis of these previous studies, we hypothesize that host physiological characteristics, such as altered immune responses during HEV infection, nutritional status, or even exposure to hepatotoxic agents or coinfections, may help explain some of the differences in pregnancy-associated morbidity and mortality seen across geographic locations and even within populations.
The immunologic changes in pregnancy, specifically a presumed shift in the Th1 and Th2 balance toward a Th2 bias, are hypothesized to be necessary to prevent rejection of the developing fetal allograft, but also alter maternal defenses against infection.14 During a “normal” pregnancy, concentrations of pro-inflammatory Th1 cytokines are reduced and production of anti-inflammatory Th2 cytokines increases over the course of pregnancy.14,15 Changes to the Th1–Th2 axis may predispose pregnant women to increased susceptibility to viral infections during the course of pregnancy.7,16 Increased susceptibility during pregnancy to viral infections, such as rubella, herpes, and human papillomavirus, has been documented.15 In addition, infectious diseases such as malaria and influenza that require Th1 responses for resolution increase in severity during pregnancy.17–19 Conversely, inflammatory diseases that are exacerbated by Th1 responses, including rheumatoid arthritis and multiple sclerosis, are mitigated during pregnancy.20–23 A caveat is that most of these studies have been limited to “Western” populations in developed country settings, which limits the generalizability of these findings to developing countries where infectious diseases are more prevalent.18,24,25 There is little prospective data that document these dramatic shifts in undernourished populations under continuous infectious insult.
Micronutrients also play vital roles in maintaining and regulating an effective immune response to pathogens. Deficiencies in single or multiple micronutrients may result in a suboptimal or, in some cases, inappropriate immune response.26 The interaction between nutritional status and host defenses against infection has been recognized for decades,27 and more recently, specific roles for individual micronutrients in immunocompetence have been elucidated.26,28 Infections influence host micronutrient metabolism, modify intake requirements, and affect the interpretation of many conventional nutritional status indicators.29 Deficiencies in zinc,30 vitamin A,31 iron,32 and vitamin D33 during pregnancy are now recognized for their potential to modulate immune function and host defense against pathogens.
Most of the current knowledge about HEV pathogenesis in pregnancy comes from studies that recruited pregnant women at the time of their presentation with an acute illness to a local health facility or were identified during large outbreak investigations.34,35 These studies have led to hypotheses that focus on the specific immunologic alterations of pregnancy as potential mechanisms that increase the risk of HEV infection and/or clinical disease. However, few studies have assessed the potential effects of antecedent maternal nutritional status and immunocompetence on risk of incident HEV infection, its timing or duration. The population-based study described here arose from a unique opportunity to prospectively explore associations between early and mid-gestation maternal micronutrient status on immunological health and risk of incident HEV infection through the early postpartum (PP) period.
Materials and Methods
Participant recruitment.
Pregnant women were recruited from the JiVitA Project site in northern Bangladesh, in the Gaibandha and Rangpur districts. These women were participating in a cluster-randomized, controlled trial testing the efficacy of multiple micronutrients on a number of pregnancy and newborn outcomes in ∼40,000 pregnant women. Women were enrolled during early pregnancy and randomized by cluster of residence to a 15-vitamin and mineral supplement or the conventional standard of care, an iron and folic acid supplement, with supplementation continued through 3 months PP.36 Enrollment started in January 2008. A 3% subsample of these women participated in a more intensive study of health and nutritional status. Blood samples were collected early in pregnancy prior to receipt of micronutrient supplements, in the third trimester (TM), and at 3 months PP. Plasma was collected after centrifugation of samples in the field laboratory, stored on liquid nitrogen, and transported to Johns Hopkins University for subsequent analysis. Women from the biological sub-study with complete longitudinal data were included for HEV testing, as part of an evaluation of infections potentially causing severe obstetric complications and mortality.8 All participants signed an informed consent to participate in pregnancy identification, the micronutrient trial, and the sub-study. Ethical oversight was provided by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board (IRB 00000570) and the Bangladesh Medical Research Council (BMRC/ERC/2007-2010 935), and the trial was registered at Clinical Trials.gov (NCT00860470).
HEV detection.
All participants in the biological sub-study who contributed three plasma samples (early pregnancy, late pregnancy, and 3 months PP) that were available as of October 2010 were tested for HEV using a reverse chronological strategy. A total 1,100 women's 3-month PP specimens were tested for anti-HEV IgG, and all positive individual's early and late pregnancy specimens were tested to identify whether seroconversion occurred in the pregnancy under study. Anti-HEV IgG testing was performed using a National Institutes of Health (NIH) in-house immunoassay to the open reading frame 2 of HEV.37,38
Women who had a signal-to-cutoff ratio (S:C) ≥ 0.85 at the PP visit were considered positive for HEV antibodies. Prevalence of HEV antibodies was determined by dividing the number of women with an S:C ≥ 0.85 by the total number of women tested at 3 months PP. Of those positive women, the women with S:C less than 0.85 at early or late pregnancy were considered to be seroconverters during the study period and classified as cases. Seroconverters were assumed to have seroconverted at the midpoint between the visit when the participant last tested negative and the visit when she tested positive. The PP samples of the HEV-IgG-positive women were then tested for HEV IgM using an NIH in-house immunoassay.37
A matched control group against which to compare seroconverters was selected from among women who were HEV negative at the PP visit, thus likely negative at each preceding time point. This control group was matched by age, parity, and sector of residence (which determined micronutrient intervention group) to the HEV seroconverters. Matching was undertaken to minimize possible confounding from non-host risk factors that may cause differential exposure to HEV, limiting our ability to resolve host-level risk factors of interest. The micronutrient intervention groups were evenly balanced across this case–control study, and the geographic matching ensured that there would be no differences in micronutrient exposure between the two groups. None of the women included in this analysis reported any hepatitis-like symptoms.
Cytokine measurement.
Plasma cytokine levels were determined by the electrochemiluminescence-based Meso Scale Discovery (MSD) immunoassay in the format of the Human Th1/Th2 10-Plex Ultra-Sensitive Kit (Meso Scale Discovery, Gaithersburg, MD) for measurement of interferon gamma (IFN-γ), interleukin 10 (IL-10), IL-12, IL-13, IL-1β, IL-2, IL-4, IL-5, IL-8, and tumor necrosis factor alpha (TNF-α) according to protocols provided by the manufacturer. In brief, 25 μL serum samples or calibrator were added to each well and incubated for 2 hours at room temperature with vigorous shaking (700 rpm) on a Jitterbug-4 Microplate Thermoshaker (Boekel Scientific, Feasterville, PA). Plates were washed three times with phosphate-buffered saline-Tween (PBS-T) and incubated with 25 μL/well antibody solution for 2 hours with vigorous shaking as described. After washing three times with PBS-T, 150 μL of 2× read buffer was added to each well. Plates were immediately read using the SECTOR Imager 4000 and data were acquired using the Discovery Workbench 3.0 software (Meso Scale Discovery). Cytokines were measured in samples that had not previously undergone any additional freeze-thaw cycles.
Micronutrient and inflammatory marker measurement.
Vitamins A (retinol) and E (α- and γ-tocopherol) were measured simultaneously using reverse-phase liquid chromatography using Waters 2795 Separation Module (Waters Corporation, Milford, MA). Peaks were detected with a photodiode array detector (Waters 2996; Waters Corporation) at 325 and 295 nm for retinol and tocopherols, respectively.39 Peak area was calculated using Empower software (Waters Corporation). Deficiency was defined as circulating retinal less than 0.7 μmol/L and α-tocopherol less than 12 μmol/L. Assays for plasma ferritin, folate, vitamin B12, and C-reactive protein (CRP) were performed on the Immulite 1000 (Siemens Diagnostics, formerly Diagnostic Products Corporation, Los Angeles, CA) chemiluminescent bench top clinical chemistry analyzer. Vitamin B6 was assessed as pyridoxal-5-phosphate by high-performance liquid chromatography with fluorescence detection (Waters 2475; Waters Corporation), calibrated against controls provided by the Centers for Disease Control and Prevention. The spectrofluorometer was set at 300 nm for excitation and 400 nm for emission. Iron stores were considered depleted when ferritin was < 15 μg/L. Folate, vitamin B6, and vitamin B12 deficiency were defined as less than 6.7 nmol/L, less than 20 nmol/L, and less than 150 pmol/L, respectively. CRP above 5 mg/L indicated the presence of inflammation.
Vitamin D was measured by using an Enzyme Immunoassay (EIA) Kit (IDS, Tyne and Wear, United Kingdom) to detect 25-hydroxy vitamin D (25(OH)2D). Deficiency was defined as 25(OH)2D less than 50 nmol/L. Transferrin receptor (TfR) was measured using an EIA from Ramco Laboratories (Stafford, TX). Microplates were read with a VersaMax microplate reader with SoftMax Pro 5.3 Software (Molecular Devices, Sunnyvale, CA). Tissue iron deficiency was defined as TfR greater than 8.3 mg/L. α-1-acid glycoprotein (AGP) was measured using a radial immunodiffusion assay (Kent Laboratories, Bellingham, WA). An AGP concentration greater than 1 g/L indicated the presence of inflammation.
An atomic absorption spectrometer (Aanalyst 800; Perkin Elmer, Waltham, MA) with autosampler (AS800) was used to analyze plasma zinc, copper, and selenium. Because of the sensitivity of the instrument, dilutions of plasma up to 1:125 were made in deionized water. Zinc, copper, and selenium standards in the range of the instrument were made using inorganic solutions (Perkin Elmer) and lyophilized serum (Seronorm; Sero AS, Billingstad, Norway), and peak area per unit concentration was repeatedly checked against the recommended peak areas as an external quality control measure. Zinc deficiency was defined as less than 8.6, 7.6, and 9.0 μmol/L at early pregnancy, late pregnancy, and 3 months PP, respectively. Copper and selenium cutoffs for deficiency were less than 11.8 μmol/L and less than 70 μg/L, respectively.
Statistical analysis.
Means and standard deviations of baseline characteristics were compared between the seroconverters and controls using Wilcoxon rank-sum tests or proportion tests, as appropriate. For cytokine concentrations below the limit of detection, half the limit of detection was imputed for the analysis. P values for the difference in median cytokine concentrations between seroconverters and controls were calculated using a Kruskall–Wallis test. Student's t test and χ2 tests were used to assess differences in mean concentrations and prevalence of micronutrient deficiencies, respectively, by case–control status. Standard cutoffs for deficiency in adults, or pregnant women where available, were used.41,42 Analysis was completed using Stata version 11 (StataCorp LP, College Station, TX)40 and SAS software version 9.3 (SAS Institute Inc., Cary, NC).
Heat maps and star plots of the cytokines were completed using Partek Genomics Suite 6.5 (Partek Inc., St. Louis, MO). The heat map was generated by log-transforming and normalizing the cytokine concentrations, with the means equal to 0 and the standard deviations equal to 1. Clustering across columns is also shown, as calculated by Euclidean analysis, with hierarchical clustering being agglomerative. For star plots representing the different stages of pregnancy, data were log-transformed and normalized for each group, with means to 0 and standard deviations to 1.
Results
Hepatitis E antibody testing.
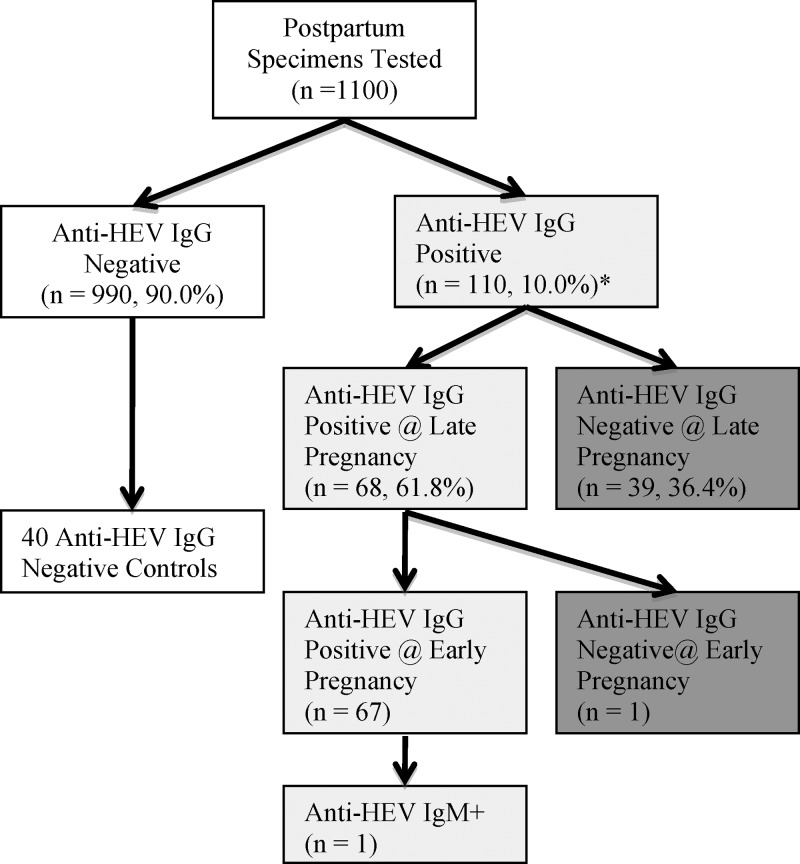
At 3 months PP, 110 (10%) of all women were positive for anti-HEV IgG (Figure 1 ). Of these 110 women, preceding early and late pregnancy samples were not available for only three, leaving 107 women to be tested for IgG antibodies during early and late pregnancy. Of those 107 women, 39 were negative at late pregnancy suggesting seroconversion occurred between late pregnancy and 3 months PP. Of the remaining 68 women positive for HEV at both late pregnancy and 3 months PP, one was negative at early pregnancy indicating that seroconversion occurred in late pregnancy, whereas the other 67 were seropositive at enrollment, suggesting a historical infection. The incidence rate of HEV seroconversion was calculated at 46 per 1,000 person-years (95% confidence interval (CI): 32–62 per 1,000 person-years). Cytokine panels and micronutrient testing was performed on the plasma of the 40 women who seroconverted during pregnancy and the PP period (one seroconversion between early and late pregnancy and 39 between late pregnancy and 3 months PP) along with 40 age-, parity-, and sector-matched controls. It is extremely salient to keep in mind that most women seroconverted between late pregnancy and 3 months PP when examining the next few sections, as significant nutritional and immunologic differences were noted in early pregnancy, well before 98% of HEV infections occurred—and that HEV infection (seroconversion) was the only characteristic used to select “cases.”
Figure 1.
Flowchart of specimen testing in pregnant women from rural Bangladesh (2007–2010). The white boxes represent hepatitis E virus (HEV)–negative women, the light gray boxes are women who were positive for HEV at baseline and likely infected before pregnancy. The dark gray boxes represent women who seroconverted during the time of this study (the cases, N = 40). Forty age-, parity-, and sector-matched controls were selected from the original negative women. Of the 110 positive specimens at 3 months postpartum, three early and late samples were not available for further testing.
Baseline characteristics.
There were no significant differences in overall demographic and socioeconomic characteristics discernable between seroconverters and controls (P > 0.05) (Table 1). A larger percentage of the women who seroconverted during pregnancy and the PP period (42.5%) had a body mass index (BMI) less than 18.5 kg/m2 than controls (25%) (P = 0.098). A larger percentage of seroconverters (35%) compared with controls (22.5%) had a mid-upper arm circumference (MUAC) less than 22 cm (a commonly used cutoff to indicate malnutrition in adults),43 but this did not reach statistical significance (P = 0.22). Distributions of the season in which pregnancies were enrolled were similar between seroconverters and controls.
Table 1.
Subject characteristics at early pregnancy assessment among seroconverters and controls in pregnant women from rural Bangladesh (2007–2010)
| Characteristic | Controls (N = 40) | Seroconverters (N = 40) | P value* |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| N | 40 | 40 | |
| Age (years)† | 22.1 (4.6) | 22.1 (4.7) | 1.00 |
| Parity† | 0.9 (0.9) | 0.9 (0.9) | 1.00 |
| Gestational age at enrollment (weeks) | 11.5 (5.1) | 10.1 (3.3) | 0.23 |
| Weight (kg) | 43.97 (6.45) | 42.96 (8.21) | 0.52 |
| Height (cm) | 148.67 (4.92) | 148.86 (4.78) | 0.94 |
| BMI (kg/m2) | 19.85 (2.49) | 19.34 (3.39) | 0.17 |
| MUAC (cm) | 24.30 (3.44) | 23.38 (3.02) | 0.21 |
| (%) | (%) | ||
| BMI ≤ 18.5 | 25.0 | 42.5 | 0.098 |
| MUAC ≤ 22 | 22.5 | 35.0 | 0.22 |
| Season‡ | |||
| Summer | 27.5 | 25.0 | 0.80 |
| Rainy | 47.5 | 55.0 | 0.50 |
| Winter | 25.0 | 20.0 | 0.59 |
BMI = body mass index; MUAC = mid-upper arm circumference; SD = standard deviation.
Wilcoxon rank-sum test for continuous variables, proportion test for categorical and binary variables.
Participants were matched on age, parity, and sector (nutritional intervention).
Summer consists of January–May, rainy of June–August, and Winter of September–December.
Cytokine analysis.
In general, the concentrations of both pro- and anti-inflammatory cytokines were higher, across all time points, in the seroconverters compared with the matched seronegative controls (Table 2). During the first TM, before the majority of documented infections occurred, median IL-12, IL-8, IL-10, and IL-4 concentrations were significantly elevated among those who subsequently seroconverted (P = 0.08, 0.008, 0.002, and 0.08, respectively). During the third TM, median IL-12 concentrations were significantly greater among seroconverters than controls (P = 0.02). At 3 months PP, IL-2 and IL-10 concentrations were elevated in the seroconverters compared with controls (P = 0.03 and 0.005, respectively). The concentrations of the remaining pro-inflammatory cytokines (i.e., IFN-γ, IL-12, IL-1β, IL-8, and TNF-α) were qualitatively higher among seroconverters than controls at 3 months PP, although not statistically significant.
Table 2.
Cytokine concentrations (pg/mL) in pregnant women from rural Bangladesh (2007–2010) at first TM, third TM, and 3 months PP by case–control status (N = 40 seroconverters and 40 controls)
| Pro-inflammatory cytokines (Th1) Median (IQR) | Anti-inflammatory cytokines (Th2) Median (IQR) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IFN-γ | IL-12 | IL-1β | IL-2 | IL-8 | TNF-α | IL-10 | IL-13 | IL-4 | IL-5 | |
| First TM | ||||||||||
| Controls | 1.94 (1.3, 3.4) | 2.43 (1.4, 4.2) | 0.31 (0.3, 0.5) | 1.69 (0.8, 2.6) | 6.85 (4.6, 8.3) | 9.54 (7.9, 11) | 2.04 (1.6, 3.3) | 1.96 (0.7, 3.3) | 0.39 (0.4, 0.9) | 2.14 (1.3, 2.9) |
| Seroconverters | 2.58 (1.6, 3.7) | 4.57 (1.9, 8.9) | 0.31 (0.3, 0.7) | 1.75 (1.2, 2.6) | 10.0 (6.4, 34) | 10.0 (8.2, 12) | 3.11 (2.3, 5.2) | 2.35 (0.7, 3.9) | 0.79 (0.4, 1.2) | 2.18 (1.4, 3.9) |
| P value* | 0.30 | 0.08 | 0.26 | 0.57 | 0.008 | 0.33 | 0.002 | 0.52 | 0.08 | 0.42 |
| Third TM | ||||||||||
| Controls | 1.84 (1.2, 3.6) | 2.55 (1.4, 3.5) | 0.71 (0.3, 1.0) | 1.75 (1.0, 2.6) | 9.62 (5.6, 18) | 9.05 (7.8, 11) | 2.41 (1.8, 3.6) | 2.14 (1.5, 3.6) | 0.89 (0.4, 1.3) | 1.38 (1.0, 2.5) |
| Seroconverters | 1.65 (1.2, 2.3) | 4.18 (2.2, 7.1) | 0.31 (0.3, 0.8) | 1.78 (0.9, 3.3) | 11.3 (8.2, 17) | 8.62 (7.6, 11) | 3.00 (1.9, 4.4) | 2.03 (0.7, 3.9) | 0.82 (0.4, 1.4) | 1.76 (1.1, 3.1) |
| P value* | 0.25 | 0.02 | 0.17 | 0.96 | 0.50 | 0.35 | 0.21 | 0.50 | 0.89 | 0.15 |
| 3 months PP | ||||||||||
| Controls | 1.73 (1.3, 3.2) | 2.90 (2.0, 5.4) | 0.31 (0.3, 0.8) | 1.94 (1.5, 2.7) | 14.1 (8.1, 23) | 10.6 (9.0, 13) | 2.13 (1.6, 3.5) | 2.20 (0.7, 3.4) | 0.39 (0.4, 0.9) | 3.23 (2.1, 4.1) |
| Seroconverters | 2.06 (1.6, 3.4) | 3.89 (2.1, 6.5) | 0.67 (0.3, 1.1) | 2.71 (1.7, 4.0) | 15.4 (8.5, 30) | 11.3 (10, 13) | 3.47 (2.2, 6.3) | 1.96 (0.7, 4.3) | 0.84 (0.4, 1.2) | 2.73 (2.0, 4.2) |
| P value* | 0.13 | 0.34 | 0.18 | 0.03 | 0.52 | 0.14 | 0.005 | 0.90 | 0.13 | 0.67 |
IFN = interferon; IL = interleukin; IQR = interquartile range; PP = postpartum; Th = T-helper cell; TM = trimester; TNF-α = tumor necrosis factor alpha.
Kruskall–Wallis test comparing seroconverters to controls.
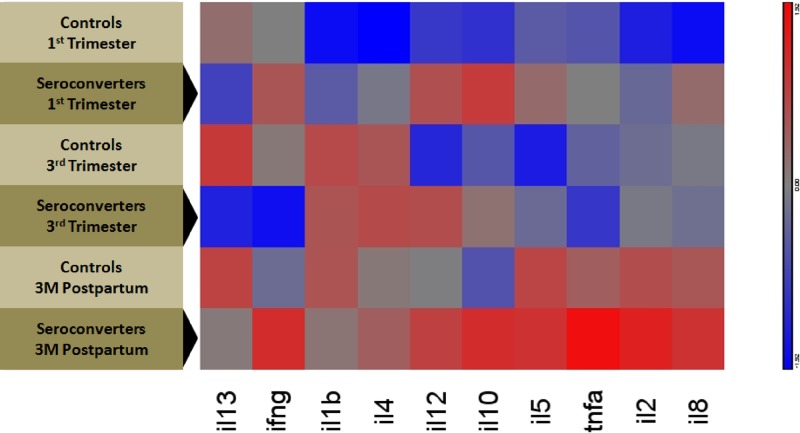
As the biological relevance of statistically significantly different concentrations of individual cytokines in a population-based study is challenging to interpret, unbiased heat maps of the normalized cytokine concentrations can be used to evaluate whether data cluster, in this study, for seroconverters and controls (Figure 2 ). The numbers of cases were also too low for more sophisticated statistical analyses to bear value. However, this also makes the differences between groups more apparent. Cytokine concentrations did not cluster along a traditional Th1/Th2 dichotomy. As suggested by the quantitative analysis, the heat map reiterated group-level upregulation of both pro- and anti-inflammatory cytokines even in early pregnancy for women who seroconverted between the third TM and 3 months PP. After the majority of seroconversions had occurred, at 3 months PP, seroconverters showed broad upregulation of nearly all cytokines measured, which was markedly different from those who remained seronegative. In addition, control women exhibited persistently lower levels of anti-inflammatory cytokines, with only a modest upregulation by 3 months PP.
Figure 2.
Heat map generated using Partek Genomics Suite 6.5 to illustrate the mean concentrations of cytokines in pregnant women from rural Bangladesh (2007–2010) at first trimester (TM), third TM, and 3 months postpartum by case–control status (N = 40 seroconverters and 40 controls). Hierarchical clustering of mean relative levels of plasma cytokine in hepatitis E virus seroconverting women and gestationally matched controls during the first TM, third TM, and at 3 months postpartum. The mean of each group was normalized to 0 and standard deviation to 1. Blue shading denotes relatively lower concentrations of a cytokine and red shading denotes relatively higher concentrations of a cytokine. The similarities of the relative changes were correlated to one another by using the Euclidean minimum distance clustering algorithm, with the results being demonstrated in the column dendrograms. All seroconversions occurred between the third TM and the 3-month postpartum visit. Here, darker red cells reflect higher concentrations, darker blue reflects relatively lower concentrations, and gray denotes no change.
To evaluate the relationships between cytokines in women who seroconverted and matched controls at each time point during pregnancy and PP (Figure 3 ), relative plasma cytokine concentrations were analyzed using star plots. The women who eventually seroconverted tended to have higher concentrations of all cytokines, whereas the controls showed lower concentrations of cytokines at all three time points. At each of the gestational times, including during the first TM (i.e., prior to HEV infection), seroconverters had higher concentrations of both pro-inflammatory cytokines (i.e., IL-12, IL-1β, IL-2, IL-8, and TNF-α) (right hemisphere) and anti-inflammatory cytokines (i.e., IL-10, IL-13, IL-4, and IL-5) (left hemisphere) when compared with controls (Figure 3). Figure 3 also suggests that the hypothesized downregulation in pro-inflammatory cytokines and upregulation in anti-inflammatory cytokines during pregnancy was not observed in this population.
Figure 3.
Star plots of normalized cytokine concentrations from pregnant women from rural Bangladesh (2007–2010) at first trimester (TM), third TM, and 3 months postpartum by case–control status (N = 40 seroconverters and 40 controls). Data are grouped by anti-inflammatory (left) and pro-inflammatory (right) cytokines. Controls are plotted in blue and seroconverters in red. Overall, hepatitis E virus (HEV) seroconversion is associated with greater cytokine concentrations during the first TM, third TM, and postpartum period.
Micronutrient analysis.
Circulating concentrations of micronutrient status indicators and the prevalence of deficiencies among seroconverters and controls are shown in Tables 3 and 4, respectively. Vitamin D deficiency was noted in 95.0% of seroconverters and 82.5% of controls in early pregnancy (P = 0.08) but did not differ at late pregnancy or PP. Serum retinol, an indicator of vitamin A status, was not significantly different between seroconverters and controls at early or late pregnancy, although it was lower among seroconverters. The prevalence of vitamin A deficiency, however, doubled among seroconverters between early pregnancy and the PP time point, while remaining unchanged among the controls. Vitamin E (α-tocopherol) deficiency did not differ between the seroconverters and controls. Circulating vitamin B6 during pregnancy was consistently lower among controls than seroconverters, although the prevalence of deficiency did not differ statistically. Circulating vitamin B12 and folate did not differ between the seroconverters and the controls.
Table 3.
Plasma micronutrient status indicators in pregnant women from rural Bangladesh (2007–2010) at first TM, third TM, and 3 months PP by case–control status
| Micronutrient | Controls (N = 40) | Seroconverters (N = 40) | P value* |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Vitamin D (nmol/L) | |||
| First TM | 41.0 (11.3) | 39.8 (5.97) | 0.57 |
| Third TM | 40.2 (11.1) | 40.6 (13.8) | 0.90 |
| 3 months PP | 41.7 (12.7) | 39.9 (9.45) | 0.48 |
| Retinol† (μmol/L) | |||
| First TM | 1.07 (0.45) | 1.04 (0.31) | 0.74 |
| Third TM | 1.20 (0.35) | 1.06 (0.33) | 0.19 |
| 3 months PP | 1.36 (0.52) | 0.94 (0.36) | 0.02 |
| α-Tocopherol† (μmol/L) | |||
| First TM | 11.5 (4.0) | 12.2 (5.0) | 0.50 |
| Third TM | 19.2 (5.1) | 19.3 (7.0) | 0.95 |
| 3 months PP | 13.3 (4.2) | 9.66 (3.8) | 0.02 |
| Vitamin B6‡ (nmol/L) | |||
| First TM | 18.3 (10.7) | 24.7 (19.7) | 0.08 |
| Third TM | 8.3 (6.3) | 12.5 (11.7) | 0.05 |
| 3 months PP | 35.5 (19.8) | 32.2 (16.0) | 0.42 |
| Vitamin B12 (pmol/L) | |||
| First TM | 205.2 (100.7) | 234.1 (120) | 0.15 |
| Third TM | 147.2 (78.5) | 176.3 (98.2) | 0.13 |
| 3 months PP | 198.4 (128) | 239.0 (131) | 0.17 |
| Folate§ (nmol/L) | |||
| First TM | 20.9 (9.9) | 21.7 (8.6) | 0.68 |
| Third TM | 27.3 (15.1) | 34.4 (21.7) | 0.10 |
| 3 months PP | 38.7 (21.9) | 39.1 (29.5) | 0.95 |
| Zinc (μmol/L) | |||
| First TM | 12.5 (2.3) | 11.5 (2.0) | 0.04 |
| Third TM | 9.72 (1.7) | 9.7 (1.8) | 0.96 |
| 3 months PP | 12.2 (3.1) | 11.0 (2.1) | 0.04 |
| Copper (μmol/L) | |||
| First TM | 22.5 (6.0) | 22.9 (6.0) | 0.80 |
| Third TM | 30.8 (7.2) | 30.9 (6.0) | 0.97 |
| 3 months PP | 21.1 (7.2) | 20.9 (5.5) | 0.90 |
| Selenium (μg/L) | |||
| First TM | 67.9 (16.1) | 67.1 (16.8) | 0.81 |
| Third TM | 66.0 (15.6) | 63.2 (14.6) | 0.42 |
| 3 months PP | 73.6 (16.4) | 67.4 (17.1) | 0.099 |
| Hemoglobin (g/L) | |||
| First TM | 121.2 (8.8) | 117.7 (11.8) | 0.13 |
| Third TM | 111.9 (7.6) | 110.7 (11.4) | 0.59 |
| 3 months PP | 122.6 (6.6) | 120.3 (9.8) | 0.22 |
| Ferritin (μg/L) | |||
| First TM | 76.3 (55.2) | 88.9 (46.8) | 0.27 |
| Third TM | 38.4 (48.2) | 48.2 (39.4) | 0.28 |
| 3 months PP | 56.8 (39.4) | 68.2 (45.1) | 0.23 |
| TfR‖ (μg/L) | |||
| First TM | 5.06 (1.53) | 4.15 (1.57) | 0.08 |
| Third TM | 5.08 (2.04) | 5.24 (2.10) | 0.82 |
| 3 months PP | – | – | – |
| CRP (mg/L) | |||
| First TM | 1.71 (2.79) | 1.58 (2.35) | 0.82 |
| Third TM | 2.42 (6.52) | 2.79 (4.49) | 0.77 |
| 3 months PP | 0.88 (1.14) | 1.95 (3.55) | 0.08 |
| AGP¶ (g/L) | |||
| First TM | 0.741 (0.26) | 0.741 (0.22) | 1.0 |
| Third TM | 0.596 (0.32) | 0.540 (0.21) | 0.36 |
| 3 months PP | 0.935 (0.28) | 0.981 (0.29) | 0.48 |
AGP = α-1-acid glycoprotein; CRP = C-reactive protein; PP = postpartum; TfR = transferrin receptor; TM = trimester; SD = standard deviation.
Student's t test comparing seroconverters versus controls.
N = 37 seroconverters at first TM; N = 26 controls and 19 seroconverters at third TM; N = 19 controls and 12 seroconverters at 3 months PP.
N = 39 seroconverters and 39 controls at first TM and 3 months PP.
N = 39 seroconverters at 3 months PP.
N = 23 controls and 15 seroconverters.
N = 39 controls at third TM.
Table 4.
Prevalence of micronutrient deficiencies over time in pregnant women from rural Bangladesh (2007–2010) at first TM, third TM, and 3 months PP by case–control status
| Micronutrient | Cutoff | Controls (N = 40) | Seroconverters (N = 40) | P value* |
|---|---|---|---|---|
| (%) | (%) | |||
| Vitamin D (nmol/L) | ||||
| First TM | < 50 nmol/L | 82.5 | 95.0 | 0.08 |
| Third TM | 77.5 | 77.5 | 1.00 | |
| 3 months PP | 82.5 | 80.0 | 0.78 | |
| Retinol† (μmol/L) | ||||
| First TM | < 0.7 μmol/L | 12.5 | 16.2 | 0.64 |
| Third TM | 7.69 | 10.5 | 0.74 | |
| 3 months PP | 10.5 | 33.3 | 0.12 | |
| α-tocopherol† (μmol/L) | ||||
| First TM | < 12 μmol/L | 62.5 | 50.0 | 0.26 |
| Third TM | 2.5 | 2.5 | 1.0 | |
| 3 months PP | 22.5 | 25.0 | 0.79 | |
| Vitamin B6‡ (nmol/L) | ||||
| First TM | < 20 nmol/L | 57.5 | 25.5 | 0.65 |
| Third TM | 95.0 | 82.5 | 0.08 | |
| 3 months PP | 12.5 | 20.0 | 0.36 | |
| Vitamin B12 (pmol/L) | ||||
| First TM | < 150 pmol/L | 40.0 | 32.5 | 0.49 |
| Third TM | 70.0 | 57.5 | 0.25 | |
| 3 months PP | 52.5 | 27.5 | 0.02 | |
| Folate§ (nmol/L) | ||||
| First TM | < 6.7 nmol/L | 0.0 | 0.0 | – |
| Third TM | 2.5 | 2.5 | 1.0 | |
| 3 months PP | 0.0 | 0.0 | – | |
| Zinc (μmol/L) | ||||
| First TM | < 8.6 μmol/L | 2.50 | 5.00 | 0.56 |
| Third TM | < 7.6 μmol/L | 5.00 | 10.0 | 0.40 |
| 3 months PP | < 9.0 μmol/L | 2.50 | 17.5 | 0.03 |
| Copper (μmol/L) | ||||
| First TM | < 11.8 μmol/L | 0.00 | 2.50 | 0.31 |
| Third TM | 0.00 | 0.00 | – | |
| 3 months PP | 2.50 | 2.50 | 1.0 | |
| Selenium (μg/L) | ||||
| First TM | < 70 μg/L | 60.0 | 67.5 | 0.49 |
| Third TM | 62.5 | 67.5 | 0.64 | |
| 3 months PP | 40.0 | 65.0 | 0.03 | |
| Hemoglobin (g/L) | ||||
| First TM | < 110 g/L | 10.0 | 27.5 | 0.045 |
| Third TM | 32.5 | 45.0 | 0.25 | |
| 3 months PP | 2.5 | 12.5 | 0.09 | |
| Ferritin (μg/L) | ||||
| First TM | < 15 μg/L | 5.0 | 2.5 | 0.56 |
| Third TM | 22.5 | 12.5 | 0.24 | |
| 3 months PP | 5.0 | 0.0 | 0.15 | |
| TfR‖ (μg/L) | ||||
| First TM | > 8.3 μg/L | 0 | 0 | – |
| Third TM | 4.35 | 6.67 | 0.75 | |
| 3 months PP | – | – | – | |
| CRP (mg/L) | ||||
| First TM | > 5 mg/L | 10.0 | 10.0 | 1.0 |
| Third TM | 7.5 | 12.5 | 0.46 | |
| 3 months PP | 2.5 | 10.0 | 0.17 | |
| AGP¶ (g/L) | ||||
| First TM | > 1 g/L | 15.0 | 10.0 | 0.46 |
| Third TM | 10.0 | 5.0 | 0.40 | |
| 3 months PP | 37.5 | 40.0 | 0.82 | |
AGP = α-1-acid glycoprotein; CRP = C-reactive protein; PP = postpartum; TfR = transferrin receptor; TM = trimester.
Chi-squared test comparing seroconverters versus controls.
N = 37 seroconverters at first TM; N = 26 controls and 19 seroconverters at third TM; N = 19 controls and 12 seroconverters at 3 months PP.
N = 39 seroconverters and 39 controls at first TM and 3 months PP.
N = 39 seroconverters at 3 months PP.
N = 23 controls and 15 seroconverters.
N = 39 controls at third TM.
Although zinc deficiency was uncommon in early pregnancy, seroconverters had significantly lower circulating zinc at the onset of their pregnancies (12.5 ± 2.3 versus 11.5 ± 2.0 μmol/L, P = 0.04) with somewhat higher prevalence of deficiency among the seroconverters when examined by cutoff (5.0% versus 2.5%, P = 0.56). This difference was substantially exacerbated by 3 months PP, with a greater percentage of seroconverters considered zinc deficient (17.5% versus 2.5%, P = 0.03). Copper deficiency was uncommon throughout pregnancy in this population. Conversely, selenium deficiency was very common, in which 60% of the controls and 67.5% of the seroconverters had low circulating selenium. The prevalence of selenium deficiency was similar between seroconverters and controls until 3 months PP when the prevalence of selenium deficiency in the seroconverters remained high at 65% but decreased to 40% in the controls (P = 0.03). Anemia, as measured by low circulating hemoglobin, occurred in nearly three times the number of seroconverters as compared with controls in early pregnancy (27.5% versus 10.0%, P = 0.045). Iron status, which is considered the most common cause of anemia during pregnancy, was rarely compromised and did not differ between the groups when assessed by plasma ferritin concentration across pregnancy (prevalence of low stores 2.5% in seroconverters versus 5.0% in controls during the first TM, P = 0.56).
Inflammation, as assessed by elevated CRP (Tables 3 and 4), occurred among approximately 10% of the women during early pregnancy and was reduced only among controls PP, with a higher prevalence of inflammation among the seroconverters, although not statistically significant. Inflammation, assessed by AGP, was not different between the seroconverters and the controls throughout pregnancy.
Discussion
In the current cohort, assessed from 2008 to 2010, starting HEV seroprevalence was 10% with an incidence rate of 46 infections per 1,000 person-years. Previously, a study of HEV seroprevalence among pregnant women from the same geographic population in northwest Bangladesh, assessed from 2001 to 2006, found a prevalence of 13.7% and an incidence rate of 56 infections per 1,000 person-years.44 The decreased incidence and prevalence could be due to improvements in hygiene and sanitation in the area, accompanying overall socioeconomic growth.13 This study, however, is the first longitudinal cohort study of HEV in pregnant women in which biospecimen were available at multiple time points prior to a documented infection. This unique opportunity allowed us to explore whether predisposing host characteristics might exist, which might differentiate pregnant women who become infected during their pregnancy and early PP period from those who remain uninfected—especially considering the similarities in risk of exposure to the virus.
The results of the cytokine analysis reveal two striking observations. First, as early as the first TM, the eventual HEV seroconverters (referred to as “cases” in this analysis) exhibited higher levels of pro- and anti-inflammatory cytokine expressions, suggesting possible a priori immunologic differences between them and women who remained seronegative. The pattern of cytokine production in early pregnancy among cases may reflect broad underlying immunologic dysfunction, predisposing these women to an increased risk of HEV infection and possibly other infections. It may also be possible that these women were infected by another pathogen, although this was not supported by their overall inflammation status. Second, robust shifts in Th1 to Th2 cytokine profiles were not observed among women in this small sample. During a “typical” pregnancy, Th1 cytokine expression tends to be downregulated, creating a Th2 bias that is commonly thought necessary for host tolerance of the growing fetus.14 Very little research has been done to characterize the “normal” immunologic trajectories during pregnancy, let alone in response to pathogens, in low-resource, high disease burden settings. More research is clearly needed to characterize this important physiologic transition, including an improved understanding of the determinants and the impacts of inadequate immunologic transition in pregnancy on the maternal-fetal dyad.
Micronutrient deficiencies may play a significant role in the observed immune dysregulation, particularly among eventual HEV seroconverters. Seroconverters had a higher proportion of women with BMI and MUAC below healthy cutoff than the controls, suggesting poorer overall nutritional status well before HEV infection. Deficiencies in immunomodulating micronutrients may predispose hosts to infection, but may also be further exacerbated by the presence of infection. In general, adequate vitamin A and D levels are required to maintain a robust anti-inflammatory Th2-biased state, while adequacy of zinc, iron, vitamin E, copper, and selenium are necessary to maintain high Th1 cytokine levels.26 Vitamin D deficiency was common in this group of women regardless of eventual seroconversion status, but seroconverters were more likely to be deficient. Insufficient vitamin D may play a role in the increased pro-inflammatory cytokines seen in the seroconverters.45 In addition, since these women have low vitamin D, macrophage function may be impaired, resulting in an increased risk of HEV infection.45 Zinc's role in promoting mucosal immunity has been well characterized.26,46 Lower zinc concentrations may increase susceptibility to HEV infection through the gut. Zinc deficiency also seems to compromise the Th1 response through reduced production of pro-inflammatory cytokines and compromising the concentration of circulating T cells.26 However, the low prevalence of zinc deficiency in this cohort may explain why we did not see lower pro-inflammatory cytokine concentrations among seroconverters. Although iron is required for the proliferation of T cells, and in particular the Th1 subset, the host sequesters iron during infections, confounding the interpretation of iron status indicators such as hemoglobin, which declines, and ferritin, which increases in the circulation when iron is sequestered.29 Although circulating hemoglobin decreased at early pregnancy in the eventual seroconverters, neither markers of iron status nor inflammation differed between the two groups. As seen with the cytokines, differences in circulating micronutrients were most notable in early pregnancy, well before seroconversions occurred. In early pregnancy, differences in case–control status in zinc, vitamin D, and hemoglobin likely reflected real differences in nutritional status at the outset of pregnancy, discerning those who would eventually be infected with HEV from those who remained uninfected. Despite known roles for other micronutrients in immune function, we did not detect differences in status in this context, possibly pointing to the importance of zinc and vitamin D in the HEV infection process.
With the exception of vitamin B6, no differences were seen between the seroconverters and controls during the third TM. This could be due to the effect of plasma volume expansion and the changing metabolic demands throughout pregnancy that lead to nutrient redistribution to support proper growth of the fetus and placenta,47 compromising the validity of conventional indicators to accurately reflect micronutrient status in late pregnancy. However, these results suggest that interventions during early pregnancy to improve micronutrient levels may possibly mediate the risk of HEV seroconversion later in pregnancy or the PP period, possibly through an immunomodulatory pathway.
This study had several limitations, notably the small sample size. The small sample size is likely to have limited our ability to detect some associations of interest. Despite the importance of the interaction between the immune system and micronutrient status, this sample was too small to examine these associations. In addition, all seroconverters in this analysis experienced a subclinical infection as none of the women reported symptoms. A future study also including clinical HEV infections is needed. Women in this biochemical sub-study were only included if they had sera at each of the three time points, possibly resulting in the exclusion of subjects who were extremely sick—this is unlikely to have substantially impacted the study as the blood draws were not scheduled based on clinical illness, but a time window in the pregnancy continuum.
This cohort provided a unique opportunity to study host factors prior to infection. Micronutrient deficiencies, from inadequate diet, and a high infectious disease burden may lead to immunologic compromise, including impaired mucosal immunity and dysregulated cytokine expression. This dysregulation in turn may lead to dysfunction of the immune system, increasing the risk of HEV infection (and perhaps the risk of other infections as well). More research is needed to replicate and explain this observed immune dysregulation. Future studies that focus on the relationship between the immune response and the clinical course of hepatitis E are needed. This study is among the first to study HEV infections in a longitudinal pregnancy cohort in a genotype 1 endemic area and may shed some light onto host-level determinants of the risk and, possibly, severity of HEV infection and hepatitis E illness. A complex series of interactions spanning host nutritional status, consequent immunocompetence, and other environmental determinants that affect nutrition and immunity are likely to be at play. For decades, we have been trying to decrypt the mystery of hepatitis E's tragic consequences to pregnant women—these data may provide a first glimpse into both preventive nutritional interventions as well as possible biomarkers of increased risk for severe disease.
ACKNOWLEDGMENTS
We thank all study participants and the field researchers and staff of the JiVitA Maternal and Child Health and Nutrition Research Project in Gaibandha, Bangladesh. We also thank Margia Arguello, Hongjie Cui, and Ashika Nanayakkara-Bind for assistance with laboratory analyses; Maithilee Mitra for her work in managing, cleaning, and supervising the field data. We recognize the leadership of Rajen Koshy at the NIH/NIAID for his support with initial forays into this research.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This work was funded by NIH R56 award number AI068813-01A2. Support was also provided by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, NIH. The field trial was supported through grant 614 (Global Control of Micronutrient Deficiency) from the Bill & Melinda Gates Foundation, Seattle, WA (Ellen Piwoz, Senior Program Officer) and through additional support from the Sight and Life Global Nutrition Research Institute, Baltimore, MD; DSM N.V., Kaiseraugst (Switzerland), Bombay (India), and Singapore formulated, prepared, and delivered in-country micronutrient premixes for supplement production and Beximco Pharmaceuticals, Ltd., Dhaka produced, bottled, labeled, and delivered tablets during the trial, both gratis; and the Ministry of Health and Family Welfare, Government of Bangladesh, Dhaka.
Authors' addresses: Brittany L. Kmush, Kerry Schulze, Lee Wu, Sucheta Mehra, Parul Christian, and Keith West Jr., Department of International Health, Johns Hopkins School of Public Health, Baltimore, MD, E-mails: bkmush1@jhu.edu, kschulz1@jhu.edu, leewu@jhmi.edu, smehra5@jhu.edu, pchrist1@jhu.edu, and kwest1@jhu.edu. Alain Labrique and Kenrad Nelson, Departments of International Health and Epidemiology, Johns Hopkins School of Public Health, Baltimore, MD, E-mails: alabriqu@gmail.com and knelson3@jhu.edu. Wei Li, Food Science and Human Nutrition, Michigan State University, East Lansing, MI, E-mail: wli@anr.msu.edu. Sabra L. Klein, Departments of Molecular Microbiology and Immunology and Biochemistry and Molecular Biology, Johns Hopkins School of Public Health, Baltimore, MD, E-mail: sklein2@jhu.edu. Saijuddin Shaikh and Hasmot Ali, The JiVitA Maternal and Child Health Research Project, Gaibandha, Bangladesh, E-mails: saiju.jivita@gmail.com and hasmot.jivita@gmail.com. Ronald E. Engle and Robert H. Purcell, Hepatitis Viruses Section, NIAID, National Institutes of Health, DHHS, Rockville, MD, E-mails: rengle@niaid.nih.gov and roberthpurcell@gmail.com.
References
- 1.Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494–503. doi: 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Khuroo MS. Hepatitis E: the enterically transmitted non-A, non-B hepatitis. Indian J Gastroenterol. 1991;10:96–100. [PubMed] [Google Scholar]
- 3.Zhuang H, Cao XY, Liu CB, Wang GM. Epidemiology of hepatitis E in China. Gastroenterol Jpn. 1991;26(Suppl 3):135–138. doi: 10.1007/BF02779283. [DOI] [PubMed] [Google Scholar]
- 4.Dalton HR, Bendall RP, Rashid M, Ellis V, Ali R, Ramnarace R, Stableforth W, Headdon W, Abbott R, McLaughlin C, Froment E, Hall KJ, Michell NP, Thatcher P, Henley WE. Host risk factors and autochthonous hepatitis E infection. Eur J Gastroenterol Hepatol. 2011;23:1200–1205. doi: 10.1097/MEG.0b013e32834ca4da. [DOI] [PubMed] [Google Scholar]
- 5.Kuniholm MH, Purcell RH, McQuillan GM, Engle RE, Wasley A, Nelson KE. Epidemiology of hepatitis E virus in the United States: results from the third National Health and Nutrition Examination Survey, 1988–1994. J Infect Dis. 2009;200:48–56. doi: 10.1086/599319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labrique AB, Kuniholm MH, Nelson KE. The global impact of hepatitis E: new horizons for an emerging virus. In: Scheld WM, Grayson ML, Hughes JM, editors. Emerging Infections. 9th edition. Washington, DC: ASM Press; 2010. pp. 53–93. [Google Scholar]
- 7.Navaneethan U, Mohajer MA, Shata MT. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int. 2008;28:1190–1199. doi: 10.1111/j.1478-3231.2008.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labrique AB, Sikder SS, Krain LJ, West KP, Jr, Christian P, Rashid M, Nelson KE. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18:1401–1404. doi: 10.3201/eid1809.120241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gurley ES, Halder AK, Streatfield PK, Sazzad HM, Huda TM, Hossain MJ, Luby SP. Estimating the burden of maternal and neonatal deaths associated with jaundice in Bangladesh: possible role of hepatitis E infection. Am J Public Health. 2012;102:2248–2254. doi: 10.2105/AJPH.2012.300749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoszek SK, Engle RE, Abdel-Hamid M, Mikhail N, Abdel-Aziz F, Medhat A, Fix AD, Emerson SU, Purcell RH, Strickland GT. Hepatitis E antibody seroconversion without disease in highly endemic rural Egyptian communities. Trans R Soc Trop Med Hyg. 2006;100:89–94. doi: 10.1016/j.trstmh.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Patra S, Kumar A, Trivedi SS, Puri M, Sarin SK. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann Intern Med. 2007;147:28–33. doi: 10.7326/0003-4819-147-1-200707030-00005. [DOI] [PubMed] [Google Scholar]
- 12.Rasheeda CA, Navaneethan U, Jayanthi V. Liver disease in pregnancy and its influence on maternal and fetal mortality: a prospective study from Chennai, southern India. Eur J Gastroenterol Hepatol. 2008;20:362–364. doi: 10.1097/MEG.0b013e3282f246d6. [DOI] [PubMed] [Google Scholar]
- 13.Labrique AB, Kmush B, Engle R, Schulze K, West KPJ, Purcell R, Nelson K. Seroincidence of Intrapartum Hepatitis E Virus Infections Declines in Pregnant Bangladeshi Women between 2001 and 2010; 3rd North American Congress of Epidemiology; Montreal, Quebec, Canada. June 22–24, 2011.2011. [Google Scholar]
- 14.Klein SL, Roberts CW, editors. Sex Hormones and Immunity to Infection. New York, NY: Springer; 2010. [Google Scholar]
- 15.Priddy KD. Immunologic adaptations during pregnancy. J Obstet Gynecol Neonatal Nurs. 1997;26:388–394. doi: 10.1111/j.1552-6909.1997.tb02720.x. [DOI] [PubMed] [Google Scholar]
- 16.Pal R, Aggarwal R, Naik SR, Das V, Das S, Naik S. Immunological alterations in pregnant women with acute hepatitis E. J Gastroenterol Hepatol. 2005;20:1094–1101. doi: 10.1111/j.1440-1746.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- 17.Luft BJ, Remington JS. Effect of pregnancy on resistance to Listeria monocytogenes and Toxoplasma gondii infections in mice. Infect Immun. 1982;38:1164–1171. doi: 10.1128/iai.38.3.1164-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J Immunol. 1996;156:644–652. [PubMed] [Google Scholar]
- 19.Menendez C. Malaria during pregnancy: a priority area of malaria research and control. Parasitol Today. 1995;11:178–183. doi: 10.1016/0169-4758(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 20.Raychaudhuri SP, Navare T, Gross J, Raychaudhuri SK. Clinical course of psoriasis during pregnancy. Int J Dermatol. 2003;42:518–520. doi: 10.1046/j.1365-4362.2003.01760.x. [DOI] [PubMed] [Google Scholar]
- 21.Ostensen M, Villiger PM. Immunology of pregnancy—pregnancy as a remission inducing agent in rheumatoid arthritis. Transpl Immunol. 2002;9:155–160. doi: 10.1016/s0966-3274(02)00017-5. [DOI] [PubMed] [Google Scholar]
- 22.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in multiple sclerosis group. N Engl J Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 23.Murphy KP, Travers P, Walport M, Janeway C. Janeway's Immunobiology. New York, NY: Garland Science; 2008. p. 887. [Google Scholar]
- 24.Piccinni MP. T cells in pregnancy. Chem Immunol Allergy. 2005;89:3–9. doi: 10.1159/000087904. [DOI] [PubMed] [Google Scholar]
- 25.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 26.Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51:301–323. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- 27.Scrimshaw NS, Taylor CE, Gordon JE. Interactions of nutrition and infection. Mongr Ser World Health Organ. 1968;57:3–329. [PubMed] [Google Scholar]
- 28.Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98((Suppl 1)):S29–S35. doi: 10.1017/S0007114507832971. [DOI] [PubMed] [Google Scholar]
- 29.Thurnham DI, Mburu AS, Mwaniki DL, De Wagt A. Micronutrients in childhood and the influence of subclinical inflammation. Proc Nutr Soc. 2005;64:502–509. doi: 10.1079/pns2005468. [DOI] [PubMed] [Google Scholar]
- 30.Shah D, Sachdev HP. Zinc deficiency in pregnancy and fetal outcome. Nutr Rev. 2006;64:15–30. doi: 10.1111/j.1753-4887.2006.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization . Global Prevalence of Vitamin A Deficiency in Populations At Risk 1995–2005. Anonymous WHO Global Database on Vitamin A Deficiency. Geneva, Switzerland: World Health Organization; 2009. [Google Scholar]
- 32.Lee AI, Okam MM. Anemia in pregnancy. Hematol Oncol Clin North Am. 2011;25:241–259. doi: 10.1016/j.hoc.2011.02.001. vii. [DOI] [PubMed] [Google Scholar]
- 33.Dror DK, Allen LH. Vitamin D inadequacy in pregnancy: biology, outcomes, and interventions. Nutr Rev. 2010;68:465–477. doi: 10.1111/j.1753-4887.2010.00306.x. [DOI] [PubMed] [Google Scholar]
- 34.Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252–255. doi: 10.1016/0002-9343(81)90758-0. [DOI] [PubMed] [Google Scholar]
- 35.Shrestha P, Bhandari D, Sharma D, Bhandari BP. A study of viral hepatitis during pregnancy in Nepal Medical College Teaching Hospital. Nepal Med Coll J. 2009;11:192–194. [PubMed] [Google Scholar]
- 36.West KP, Jr, Shamim AA, Mehra S, Labrique AB, Ali H, Shaikh S, Klemm RD, Wu LS, Mitra M, Haque R, Hanif AA, Massie AB, Merrill RD, Schulze KJ, Christian P. Effect of maternal multiple micronutrient vs iron-folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: the JiVitA-3 randomized trial. JAMA. 2014;312:2649–2658. doi: 10.1001/jama.2014.16819. [DOI] [PubMed] [Google Scholar]
- 37.Yu C, Engle RE, Bryan JP, Emerson SU, Purcell RH. Detection of immunoglobulin M antibodies to hepatitis E virus by class capture enzyme immunoassay. Clin Diagn Lab Immunol. 2003;10:579–586. doi: 10.1128/CDLI.10.4.579-586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engle RE, Yu C, Emerson SU, Meng XJ, Purcell RH. Hepatitis E virus (HEV) capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by enzyme immunoassay. J Clin Microbiol. 2002;40:4576–4580. doi: 10.1128/JCM.40.12.4576-4580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamini S, West KP, Jr, Wu L, Dreyfuss ML, Yang DX, Khatry SK. Circulating levels of retinol, tocopherol and carotenoid in Nepali pregnant and postpartum women following long-term beta-carotene and vitamin A supplementation. Eur J Clin Nutr. 2001;55:252–259. doi: 10.1038/sj.ejcn.1601152. [DOI] [PubMed] [Google Scholar]
- 40.StataCorp . Stata Statistical Software. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 41.Schulze KJ, Christian P, Wu LS, Arguello M, Cui H, Nanayakkara-Bind A, Stewart CP, Khatry SK, LeClerq S, West KP., Jr Micronutrient deficiencies are common in 6- to 8-year-old children of rural Nepal, with prevalence estimates modestly affected by inflammation. J Nutr. 2014;144:979–987. doi: 10.3945/jn.114.192336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang T, Christian P, Khatry SK, Wu L, West KP., Jr Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. J Nutr. 2005;135:1106–1112. doi: 10.1093/jn/135.5.1106. [DOI] [PubMed] [Google Scholar]
- 43.James WP, Mascie-Taylor GC, Norgan NG, Bistrian BR, Shetty PS, Ferro-Luzzi A. The value of arm circumference measurements in assessing chronic energy deficiency in Third World adults. Eur J Clin Nutr. 1994;48:883–894. [PubMed] [Google Scholar]
- 44.Labrique AB, Kuniholm M, Engle RE, Rashid M, Ali H, Nelson KE. Seroprevalence of Antibodies to Hepatitis E Virus (HEV) and HEV Incidence in Pregnant Women in Northwest Rural Bangladesh. 13th International Symposium on Viral Hepatitis and Liver Disease; Washington, DC. March 20–24, 2009.2009. [Google Scholar]
- 45.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–1720S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 46.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–298. doi: 10.1146/annurev.nutr.24.012003.132454. [DOI] [PubMed] [Google Scholar]
- 47.FAO/WHO/UNU Expert Consultation . Human Energy Requirements. Rome, Italy: FAO; 2001. [Google Scholar]