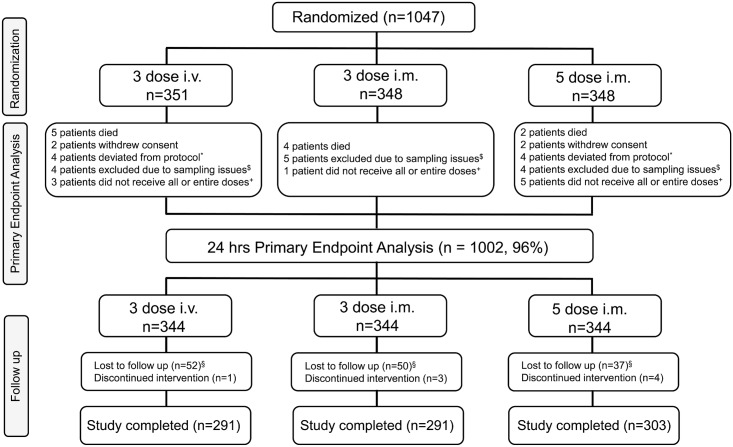
Fig 1. Trial profile.
*These patients completed the study but were not included for the primary endpoint analysis because of protocol deviations. $These patients completed the study but were not included for the primary endpoint analysis because of sampling issues. +These patients completed the study but were not included for the primary endpoint analysis because of dosing issues. §Lost to follow-up includes patients who (i) withdrew consent (n = 8), (ii) moved from the study area (n = 9), and (iii) were discharged from the study due to malaria infection on day 28 (n = 1), amongst a variety of other reasons.