Abstract
With limited availability of osteochondral allografts, tissue engineered cartilage grafts may provide an alternative treatment for large cartilage defects. An effective storage protocol will be critical for translating this technology to clinical use. The purpose of this study was to evaluate the efficacy of the Missouri Osteochondral Allograft Preservation System (MOPS) for room temperature storage of mature tissue engineered grafts, focusing on tissue property maintenance during the current allograft storage window (28 days). Additional research compares MOPS to continued culture, investigates temperature influence, and examines longer-term storage. Articular cartilage constructs were cultured to maturity using adult canine chondrocytes, then preserved with MOPS at room temperature, in refrigeration, or kept in culture for an additional 56 days. MOPS storage maintained desired chondrocyte viability for 28 days of room temperature storage, retaining 75% of the maturity point Young's modulus without significant decline in biochemical content. Properties dropped past this time point. Refrigeration maintained properties similar to room temperature at 28 days, but proved better at 56 days. For engineered grafts, MOPS maintained the majority of tissue properties for the 28-day window without clearly extending that period as it had for native grafts. These results are the first evaluating engineered cartilage storage.
Keywords: preservation, storage, tissue engineering, articular cartilage
Articular cartilage defects greater than 2 cm2 are often treated with osteochondral allografts. Although fresh grafts have been shown to result in long-term functional outcomes,1 success is a function of the viability of the graft's chondrocytes, which diminishes over storage time1–4 leading surgeons to consider 28 days post-harvest as the limit for the optimal transplantation window. Current mandatory disease testing of fresh grafts takes at least 14 days, which limits graft availability for clinical use to 14 days or less.5 Current tissue bank storage protocols entail grafts being stored in fetal bovine serum (FBS)-containing media in refrigeration (4°C).6 Based on the limitations of current storage protocols with respect to graft availability, researchers are investigating various methods of storage in an attempt to increase the duration of this storage window.6–12 A preservation system recently developed by Stoker et al., called the Missouri Osteochondral Allograft Preservation System (MOPS), has shown maintenance of day 0 chondrocyte viability for at least 63 days without the use of serum, growth factors, or refrigeration.13,14 This preservation system reduces concerns associated with xenogenic media components and simplifies storage logistics.
Still, osteochondral allografts are in insufficient supply to meet clinical demand.15,16 Tissue engineering may offer an alternative, cell-based strategy for fabricating biomimetic grafts.17 Engineered tissue grafts have been fabricated and cultured to reach native or near-native mechanical and biochemical properties.18–21 To facilitate eventual clinical translation, engineered grafts will also require an effective preservation/storage protocol, in order to give the product a “shelf-life”.22 As native and engineered cartilage differ in structure and composition, preservation requirements of the tissues may also differ.
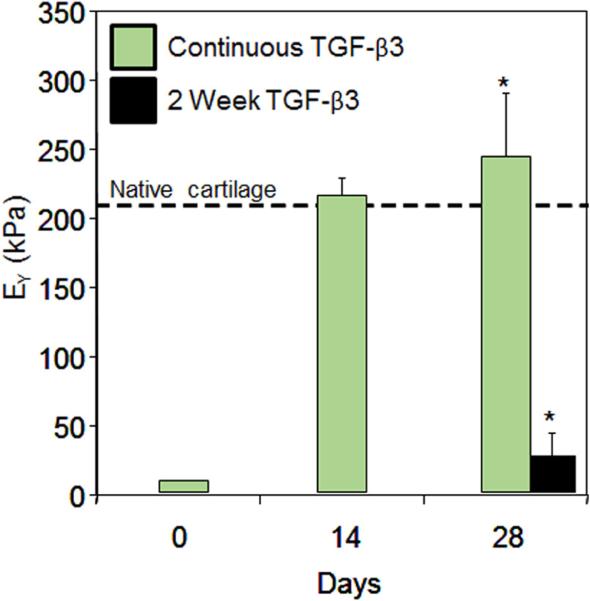
Previous studies in our laboratory have shown that engineered tissues grown with adult cells require culture media supplementation with transforming growth factor-β3 (TGF-β3) for development and maintenance of tissue properties.18,23,24 Cessation of growth factor administration after 14 days (e.g., transient growth factor exposure), an effective protocol for promoting functional development of engineered constructs derived from juvenile chondrocytes,24–27 was shown to decrease Young's modulus of mature-cell constructs to ~15% of original values in only two weeks (day 28 of culture)24 (Fig. 1). As the MOPS system does not include TGF-β3, we speculated that engineered tissues would exhibit a similar loss of tissue properties under the prescribed preservation conditions.
Figure 1.
Young's Modulus (EY, n = 4–5) of constructs engineered with Passage 1 adult canine chondrocytes cultured with continuous (Day 0–28) or transient (Day 0–14) TGF-β3 supplementation. *indicates p < 0.05 difference between groups at day 28. Dashed line indicates native Young's modulus of canine cartilage. Adapted from ref.24
In the current study, MOPS preservation of tissue engineered cartilage grafts, cultivated to native mechanical properties, was investigated to determine its suitability for this purpose. We explored its efficacy by comparing grafts maintained in culture as well as at room temperature and cold storage. While the immediate focus of this evaluation was on the critical clinical window of 28 days, preservation up to 56 days was investigated to explore the potential for longer-term storage. Constructs were evaluated for mechanical, biochemical, histological, gross, and viability properties. Tissue properties were compared to those at the point of maturation and compared to the other experimental groups at each time point.
MATERIALS AND METHODS
Sample Preparation and Tissue Culture
Articular cartilage chunks were harvested from adult canine knees,19,21 then digested in 390 U/ml collagenase type IV (Worthington, Lakewood, NJ) for 8 h with slight agitation. Isolated primary chondrocytes were then passaged once (P1) or twice (P2) in Dulbecco's Modified Eagle's Media (DMEM, Invitrogen, Carlsbad, CA) containing 10% FBS (Atlanta Biologicals, Norcross, GA), 1 ng/ml transforming growth factor-beta-1 (TGF-β1, Invitrogen) and 5 ng/ml fibroblast growth factor-2 (FGF-2, Invitrogen), and 1% antibiotics/anti-mycotics (Invitrogen). Chondrocytes were encapsulated at 30 × 106 cells/mL in 2% w/v agarose (Type VII, Sigma–Aldrich, St. Louis, MO), and cast between parallel plates. Cylindrical disks were punched from this slab, yielding constructs of 4.0 mm diameter and 2.3 mm thickness. Constructs were cultured in chemically defined media (chondrogenic media, CM) consisting of DMEM containing 50 μg/mL l-proline (Sigma–Aldrich), 100 μg/mL sodium pyruvate (Sigma–Aldrich), 1% ITS+ premix (BD Biosciences, San Jose, CA), 100 nM dexamethasone (Sigma–Aldrich), 1% antibiotics/antimycotics, 50 μg/mL ascorbic acid (Sigma–Aldrich), and 10 ng/ml TGF-β3 (R&D Systems, Minneapolis, MN) with media changes three times per week. Constructs were evaluated on biweekly time points.
Preservation/Storage
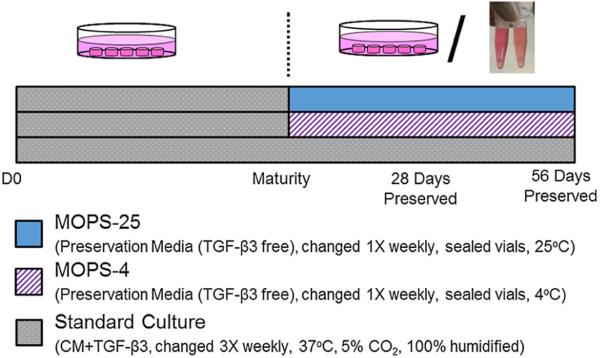
Once constructs reached the point of maturity (28–42 days), judged by an average equilibrium compressive Young's Modulus (EY) in the native range (> 200 kPa 18), constructs were randomly assigned to experimental groups: MOPS-25, standard preservation in MOPS media at room temperature (25°C)13,14; MOPS-4, preservation in MOPS media with refrigeration (4°C); or continuous culture, in which constructs remained in the culture conditions described above. For constructs in MOPS media, the media were changed once per week and did not contain growth factors or FBS.2,13,14,18 MOPS media components include DMEM, antibiotics, antimycotics, dexamethasone, ascorbic acid, l-proline, sodium pyruvate, insulin, transferrin, and selenous acid. The maturity point served as the baseline for preservation. Culture and preservation of experimental groups is summarized in Figure 2.
Figure 2.
Schematic of experimental group conditions in the documented studies. Maturity was judged by EY > 200 kPa.
Mechanical Testing
Spatially averaged mechanical properties were evaluated using custom testing device as previously described.28 EY was assessed from the steady-state response under 10% unconfined compressive strain. The dynamic modulus (G*) was evaluated at 0.01 Hz and 1% strain amplitude, superimposed over the steady-state response under 10% compressive strain.
Biochemical Analysis
Constructs were weighed wet (WW) and, following lyophilization, dry (DW), then digested in 0.5 mg/mL proteinase-K (MP Biomedicals, Santa Ana, CA) for 16 h at 56°C. Full constructs were used for biochemical analyses for all time points other than day 0 (initial casting), at which half constructs were used. DNA content was measured using a PicoGreen assay (Invitrogen) with lambda phage DNA standards.29 Glycosaminoglycan (GAG) content was measured using a 1,9-dimethylmethylene blue (Sigma–Aldrich) dye-binding assay with shark chondroitin-6-sulfate (Sigma–Aldrich) standards.30 An aliquot of the proteinase-K digested solution was hydrolyzed in 12 N HCl at 110°C for 16 h, dried, and then resuspended in assay buffer.31 Orthohydroxyproline (OHP) content was then measured by a colorimetric assay scaled to microplates involving reactions with chloramine T and dimethylaminobenzaldehyde.31 Collagen content was calculated by assuming a 1:7.64 OHP-to-collagen mass ratio.32 Biochemical quantities were analyzed as absolute values as well as normalized to WW, DW, and DNA content. For preserved groups, media from weekly changes were saved, and assayed for GAG content.
Cell Viability and Metabolism
Constructs were halved, stained with a LIVE/DEAD® Assay Kit (Invitrogen), and their cross-section imaged using a confocal microscope (Olympus Fluoview FV1000, Waltham, MA). To measure metabolism, individual constructs were incubated in 1 ml 0.5 mg/mL MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide, Sigma–Aldrich) in MOPS media for 3 h, then removed, minced, and placed in a dimethyl sulfoxide/isopropanol solution; dye absorbance was measured on a microplate reader at 670 nm. Absorbance values were normalized to the average absorbance at the maturation point.
Histological Analysis
Constructs were fixed in 5% acetic acid, 3.7% formaldehyde, 70% ethanol solution for 24 h, then stored in 70% ethanol.33 Constructs were serially dehydrated in ethanol and embedded in paraffin (Fisher Scientific, Waltham, MA). Then, samples were sectioned to 8 μm and mounted on glass slides. Samples were dewaxed, rehydrated, and stained with Alcian Blue (Sigma–Aldrich) and Picrosirius Red (Sigma–Aldrich) to visualize GAG and collagen distribution, respectively. Full constructs were used for biochemical analyses for all time points other than day 0 (initial casting), at which half constructs were used.
Statistical Analysis and Sample Size
Statistica (Statsoft, Tulsa, OK) was used to perform statistical analyses. Two-way analysis of variance (ANOVA) tests were utilized, followed by a Tukey honest significant difference (HSD) post-hoc test (α = 0.05) to determine statistical significance (p ≤ 0.05).
Separate studies comparing MOPS-25 to continued control, and comparing MOPS-25 to MOPS-4, were run and repeated (MOPS-25: 4 individual runs, MOPS-4, Continued Culture: 2 individual runs). For each time point, sample size per group was at minimum three (n ≥ 3). Here, for easy visualization and cross-study analysis, results from each study were normalized to the respective maturity point average value, then pooled.
RESULTS
Constructs reached maturity (> 200 kPa) in 28–42 days, providing a baseline for preservation. Averaged across the four studies, the maturity point tissue properties were: EY = 258 ± 23 kPa, G* = 1.68 ± 0.09 MPa, GAG = 1.97 ± 0.06 mg (4.93 ± 0.17% wet weight), and collagen= 0.70 ± 0.05 mg (1.73 ± 0.13% wet weight). Following this maturity point, the constructs were split into the experimental groups. Time points refer to the days post-maturity, either stored/preserved or kept in culture.
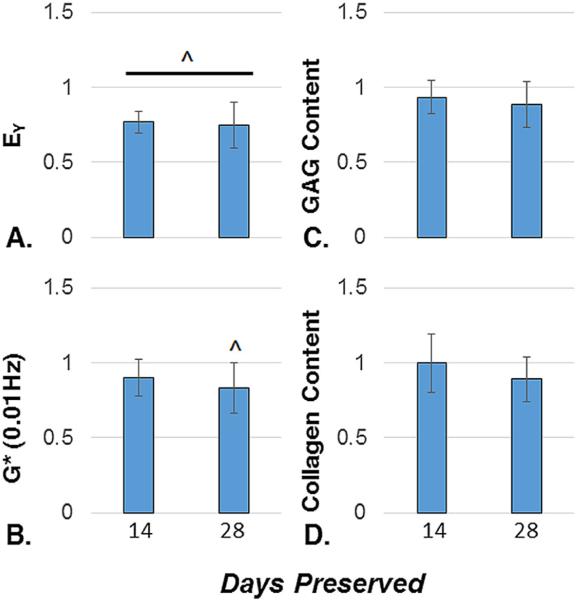
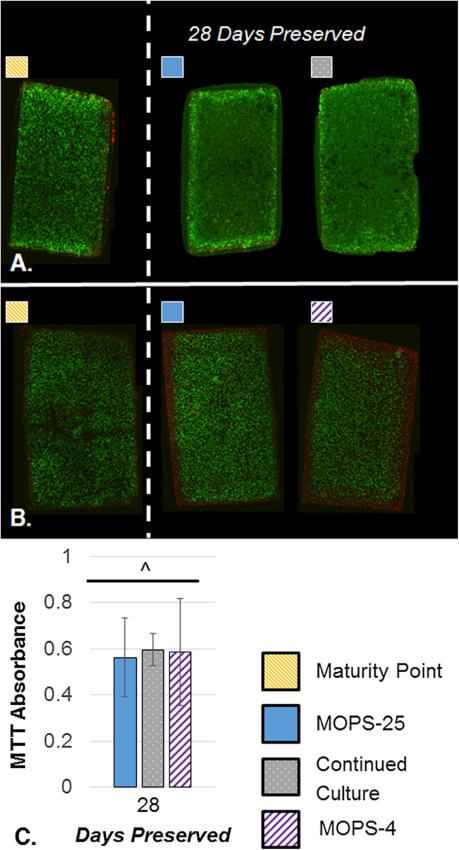
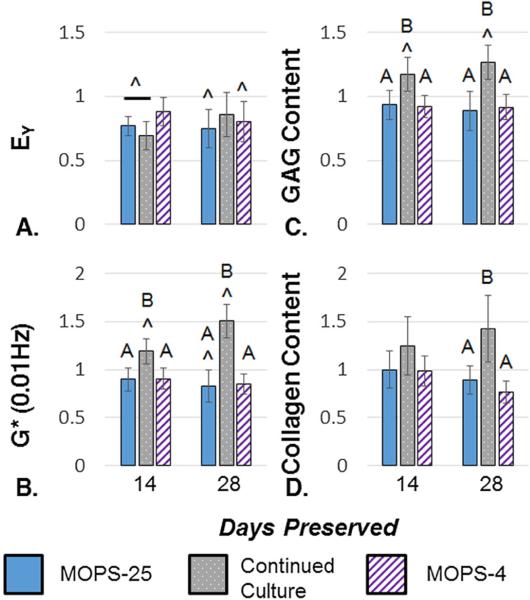
At the end of the critical clinical window of 28 days, the MOPS-25 protocol maintained 74.7 ± 15.1% of EY, 83.2 ± 16.6% of G*, 88.6 ± 15.6% of the total GAG content, and 89.1 ± 14.7% of the total collagen content averaged across four independent studies (Fig. 3). In that window, GAG and collagen content did not drop significantly, whereas EY and G* were significantly lower than the maturity point. Viability staining showed the majority of cells to be viable following 28 days of preservation (Fig. 4 A and B). MTT data showed the metabolic activity decreased over time, with 56.2 ± 17.1% of baseline metabolic activity at 28 days of storage (Fig. 4C).
Figure 3.
A–D. Young's Modulus (n = 23–25) (A), Dynamic Modulus (n = 24–25) (B), GAG Content (n = 17–19) (C), and Collagen Content (n = 16–21) (D) normalized to respective maturity point tissue properties (n = 26–32), and averaged across four independent studies for samples preserved for 14 and 28 days with MOPS-25. ^indicates significant difference from maturity point (p < 0.05).
Figure 4.
A–C. Representative cross-sectional Live/Dead images comparing MOPS-25 to Continued Culture and Maturity Point (A) and comparing MOPS-25 to MOPS-4 and Maturity Point (B) at 28 days preserved (n = 2–4). MTT absorbance values after 28 days preserved normalized to maturity point values (C). MTT values for MOPS-25 are pooled from three independent studies (n = 12), values for CC are from one independent study (n = 3), and values for MOPS-4 are pooled from two independent studies (n = 8). Maturity point data pooled from three independent studies (n = 14). ^indicates significant difference from maturity point (p < 0.05). There is no significant difference between groups.
Continuing culture of constructs led to significantly decreased EY at 14 days past maturity, which was not significantly lower than the maturity point at 28 days past maturity (Fig. 5). However, G* and total GAG content increased significantly over the maturity point and the preserved groups (Fig. 5). Total collagen content also increased, becoming significantly different than the preserved groups (Fig. 5). Viability was not different from MOPS preservation (Fig. 4A).
Figure 5.
A–D. Young's Modulus (n = 10–25) (A), Dynamic Modulus (n = 10–25) (B), GAG Content (n 9–19) (C), and Collagen Content (n = 9–21) (D) normalized to respective maturity point tissue properties (n = 26–32), and averaged across 2 (Continued Culture, MOPS-4) or 4 (MOPS-25) independent studies for samples preserved for 14 and 28 days with. ^indicates significant difference from maturity point and letters indicate significantly different groups (p < 0.05).
MOPS-4 showed no difference compared to MOPS-25 in the first 28 days (Fig. 5). Viability and metabolism were not different from MOPS preservation (Fig. 4B and C).
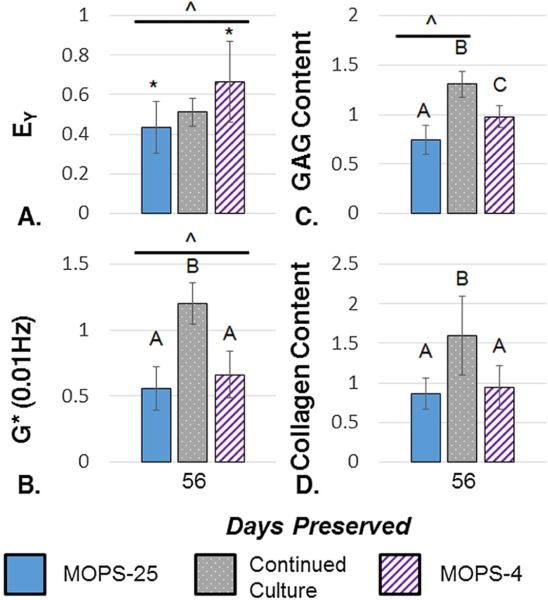
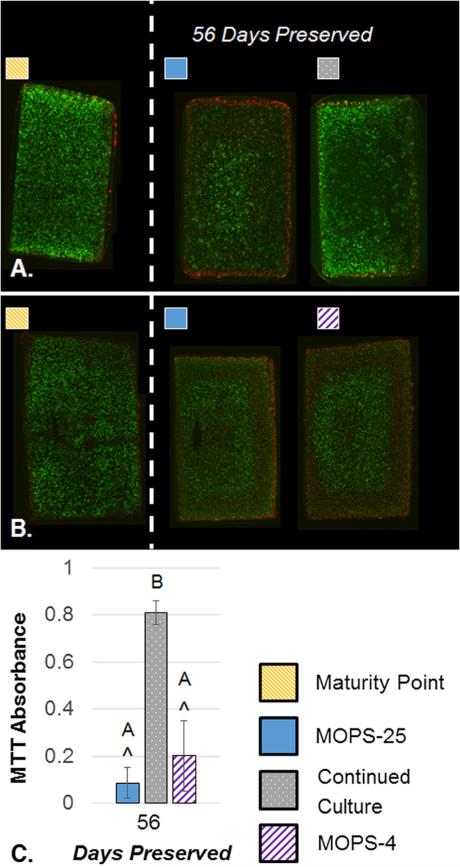
Past the 28-day clinical window, all construct properties decreased. At 56 days, constructs preserved with MOPS-25 fell to 43.4 ± 13.1% of EY, 55.9 ± 16.6% of G* yet maintained 74.1 ± 14.5% of GAG and 86.7 ± 19.3% of collagen (Fig. 6). Here, refrigeration retained significantly more GAG, leading to a significantly higher EY than the MOPS-25. In each study, GAG measured in the media was generally higher for MOPS-25 preserved constructs than MOPS-4 (data not shown). Continuing culture also led to a drop in EY, yet at 56 days past maturity G*, GAG content, and collagen content were all higher than at the maturity point. In preserved groups, the change in Young's and dynamic moduli were significantly lower than the maturity point. GAG content was significantly lower for MOPS-25 samples and significantly higher for continued culture samples compared to the maturity point. Collagen levels were not significantly different from the maturity point at 56 days past maturity. Additionally, metabolism was significantly higher for continuous culture than preserved groups (Fig. 7). Histology (not shown) corroborated trends ascertained through assays.
Figure 6.
A–D. Young's Modulus (n = 12–30) (A), Dynamic Modulus (n = 12–31) (B), GAG Content (n 8–28) (C), and Collagen Content (n = 9–26) (D) normalized to respective maturity point tissue properties (n = 26–32), and averaged across 2 (Continued Culture, MOPS-4) or 4 (MOPS-25) independent studies for samples preserved for 56 days with. ^indicates significant difference from maturity point, letters and * indicate significantly different groups (p < 0.05).
Figure 7.
A–C. Representative cross-sectional Live/Dead images comparing MOPS-25 to Continued Culture and Maturity Point (A) and comparing MOPS-25 to MOPS-4 and Maturity Point (B) at 56 days preserved (n = 2–4). MTT absorbance values after 56 days preserved normalized to maturity point values (C). MTT values for MOPS-25 are pooled from three independent studies (n = 11), values for CC are from one independent study (n = 4), and values for MOPS-4 are pooled from two independent studies (n = 8). Maturity point data pooled from three independent studies (n = 14). ^ indicates significant difference from maturity point (p < 0.05). There is no significant difference between groups.
DISCUSSION
As the field of articular cartilage tissue engineering advances, it will require an effective storage/preservation protocol to give fabricated replacement tissues a “shelf-life” prior to transplantation, allowing for proper translation into clinical use. Currently, the optimal transplantation window for organ donor osteochondral allografts effectively ends at 28 days post-procurement due to decreasing chondrocyte viability.5 As such, a storage protocol for engineered tissues should maintain properties for at least 28 days. As the MOPS protocol has been shown to maintain allograft viability for at least 63 days (35 day extension of the clinical transplantation window) with logistical, chemical, and financial benefits,13,14 it was adopted as a starting point for preservation of engineered grafts. The properties at construct maturity point (when preservation was initiated) were used as baseline from which relative changes in tissue properties could be assessed. As another point of comparison, a tissue culture group was added, representing an alternative scenario in which grafts would be maintained in tissue culture until implantation. Additionally, altering the MOPS temperature to 4°C served to investigate the influence of temperature.
Contrary to our experience growing engineered cartilage derived from adult chondrocytes that demonstrated a dramatic drop (to ~15% of initial values, Fig. 1) in tissue properties upon withdrawal of growth factor supplementation of the media,24 application of growth factor-free MOPS to mature tissue engineered cartilage was observed to maintain most tissue properties within the critical clinical window, with mostly viable cells after 28 days (Fig. 4). EY and G* dropped significantly to 70% of their value at the maturity point. Still, GAG and collagen content were not significantly changed from the maturity point (Fig. 3). While metabolism decreased, this is common for grafts stored under sub-physiologic temperatures.34 These encouraging results suggest that MOPS has potential for storing engineered cartilage for up to 28 days.
Whereas MOPS has shown to extend maintenance of native canine allografts to 63 days of storage,14 it did not provide similar results with engineered tissues. At 56 days of storage, EY, G*, and GAG content were significantly lower than their maturity point values, though collagen content remained insignificantly changed (Fig. 6). At 56 days preserved, chondrocyte viability decreased, yet still displayed viable cells in each independent study (Figs. 4 and 7). In samples preserved for 56 days (Fig. 7), viability staining revealed an unusual pattern consisting of brightly stained dead cells on the immediate periphery, brightly stained live cells in the center, and a mix of dead cells and dimly stained live cells in between. This pattern may be related to susceptibility of the phenotype to pathology, the breakdown of extracellular matrix, a chemical factor released by the cells into the media, and/or the lower than physiologic storage temperature. Future studies will investigate the cause of this unique arrangement.
In terms of temperature, refrigeration was insignificantly different than room temperature at 28 days preserved. However, temperature may be more important with longer storage times, as refrigeration led to EY and GAG content that was significantly higher than MOPS-25 at 56 days preserved (Figs. 5 and 6). The GAG content in the media implies decreased turnover and metabolism in refrigerated samples. Starting when placed in preservation conditions, engineered tissues decrease in metabolic activity over time as the properties slowly decrease, until the cells are quiescent. These results indicate that refrigeration is preferred for the storage of tissue engineered grafts, retaining tissue properties for a longer period of time. However, within the 28 day clinical transplant window, room temperature storage may be satisfactory, and logistically easier.
These studies have several limitations. While the adult canine model is an excellent preclinical transplantation model,18,35–37 it still does not exactly replicate the adult human clinical situation. However, repeated success culturing canine chondrocytes in agarose to native or near-native properties18,19,21 makes it a robust model system for the current studies. Similarly, these studies are purely in vitro, aiming to maintain tissue properties over time. An in vivo study would be required to fully understand how preserved engineered grafts will fare after transplantation. The continued culture group presented for reference is not a realistic clinical scenario: It requires greater cost and labor (3× weekly media change with growth factor supplementation, incubation), and would still require storage during mandatory disease/contamination screening. Nevertheless, this is a valuable reference point, providing knowledge of tissue properties past maturity which may plateau or decline without additional stimulation, such as dynamic loading.19 Additionally, unlike clinical allografts, the tissue engineered constructs in these studies are not osteochondral; however, even for clinical allografts, the bone portion is treated as nonliving,38 and so it is believed that the results of these studies, preserving chondral-only engineered grafts will be applicable to preserving tissue engineered osteochondral grafts, which, likewise, incorporate nonliving bone or bone-like regions.39
While, to our knowledge, no literature currently exists concerning the storage of tissue engineered cartilage grafts, there have been many clinical and basic science studies assessing stored grafts in vivo3,4,11–13,40–42 and in vitro6–10,14,34,40,43–49 using a variety of storage protocols and evaluation methods. Most commonly, chondrocyte viability is used as the marker of allograft success,3 yet ideally tissue properties best match freshly harvested tissues, which have been shown to have maximum viability and long-term implantation success.1,5,38 Tissue engineering studies allow for the fabrication of a high quantity of parallel samples, allowing for consistent evaluation of tissue properties. These comprehensive measurements demonstrate that MOPS can maintain the majority of tissue properties over 28 days of storage, though further optimization is needed to achieve full tissue maintenance through 28 days and beyond. Looking forward, we conceive of the ability to screen cells/constructs during construct fabrication/culturing period as is done routinely for commercially-available engineered skin.50–53 In this scenario, MOPS would further increase the clinical shelf-life of engineered OCAs, starting at the “day 0” of target mature properties. The disparate preservation response of native and engineered cartilage to MOPS may ultimately reflect inherent differences between chondrocytes residing in situ and those manipulated in culture, and directed toward de novo tissue formation. Future studies will include alterations of the storage protocol to decrease degradation and an in vivo study to ensure the translational efficacy of stored engineered grafts.
ACKNOWLEDGMENTS
We thank Daniel R. Howard, M.D., Rebecca A. Peyser, and William T. Yu for their additional help on this project. JLC, AMS, CTH are patent holders of the Missouri Osteochondral Allograft Preservation System used in this study and will receive royalties for future clinical use.
Research was performed at the Cellular Engineering Laboratory at Columbia University, New York, NY, USA.
Grant sponsor: The Coulter Foundation; Grant number: NIH 2R01AR046568; Grant sponsor: Columbia Technology Ventures; Grant number: NIH 1R01AR060361; Grant sponsor: National Institutes of Health; Grant number: NIH 1S10RR027943.
Footnotes
AUTHORS’ CONTRIBUTIONS
All authors contributed significantly to the work reported in this manuscript. ABN and CTH designed the experiments. ABN, RMS, and SLL carried out the experiments and collected the data. ABN, RMS, and CTH analyzed the data. CTH, GAA, AMS, and JLC supervised and advised the work. ABN, RMS, CTH, GAA, and JLC wrote the paper. All authors have read and approved the final submitted manuscript.
REFERENCES
- 1.Gross AE, Kim W, Las Heras F, et al. Fresh osteochondral allografts for posttraumatic knee defects: long-term followup. Clin Orthop Relat Res. 2008;466:1863–1870. doi: 10.1007/s11999-008-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaver RJ, Mahomed M, Backstein D, et al. Fresh osteochondral allografts for post-traumatic defects in the knee. A survivorship analysis. J Bone Joint Surg Br. 1992;74:105–110. doi: 10.1302/0301-620X.74B1.1732235. [DOI] [PubMed] [Google Scholar]
- 3.Pallante AL, Chen AC, Ball ST, et al. The in vivo performance of osteochondral allografts in the goat is diminished with extended storage and decreased cartilage cellularity. Am J Sports Med. 2012;40:1814–1823. doi: 10.1177/0363546512449321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bakay A, Csonge L, Papp G, et al. Osteochondral resurfacing of the knee joint with allograft. Clinical analysis of 33 cases. Int Orthop. 1998;22:277–281. doi: 10.1007/s002640050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherman SL, Garrity J, Bauer K, et al. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22:121–133. doi: 10.5435/JAAOS-22-02-121. [DOI] [PubMed] [Google Scholar]
- 6.Williams RJ, 3rd, Dreese JC, Chen CT. Chondrocyte survival and material properties of hypothermically stored cartilage: an evaluation of tissue used for osteochondral allograft transplantation. Am J Sports Med. 2004;32:132–139. doi: 10.1177/0095399703258733. [DOI] [PubMed] [Google Scholar]
- 7.Teng MS, Yuen AS, Kim HT. Enhancing osteochondral allograft viability: effects of storage media composition. Clin Orthop Relat Res. 2008;466:1804–1809. doi: 10.1007/s11999-008-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csonge L, Bravo D, Newman-Gage H, et al. Banking of osteochondral allografts, part II. preservation of chondrocyte viability during long-term storage. Cell Tissue Bank. 2002;3:161–168. doi: 10.1023/A:1023687419152. [DOI] [PubMed] [Google Scholar]
- 9.Ball ST, Amiel D, Williams SK, et al. The effects of storage on fresh human osteochondral allografts. Clin Orthop Relat Res. 2004;418:246–252. doi: 10.1097/00003086-200401000-00043. [DOI] [PubMed] [Google Scholar]
- 10.Bian L, Stoker AM, Marberry KM, et al. Effects of dexamethasone on the functional properties of cartilage explants during long-term culture. Am J Sports Med. 2010;38:78–85. doi: 10.1177/0363546509354197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaPrade RF, Botker J, Herzog M, et al. Refrigerated osteoarticular allografts to treat articular cartilage defects of the femoral condyles. A prospective outcomes study. J Bone Joint Surg Am. 2009;91:805–811. doi: 10.2106/JBJS.H.00703. [DOI] [PubMed] [Google Scholar]
- 12.Williams RJ, 3rd, Ranawat AS, Potter HG, et al. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89:718–726. doi: 10.2106/JBJS.F.00625. [DOI] [PubMed] [Google Scholar]
- 13.Cook JL, Stoker AM, Stannard JP, et al. A novel system improves preservation of osteochondral allografts. Clin Orthop Relat Res. 2014;472:3404–3414. doi: 10.1007/s11999-014-3773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoker A, Garrity JT, Hung CT, et al. Improved preservation of fresh osteochondral allografts for clinical use. J Knee Surg. 2012;25:117–125. doi: 10.1055/s-0032-1319809. [DOI] [PubMed] [Google Scholar]
- 15.Paige KT, Vacanti CA. Engineering new tissue: formation of neo-cartilage. Tissue Eng. 1995;1:97–106. doi: 10.1089/ten.1995.1.97. [DOI] [PubMed] [Google Scholar]
- 16.Mow VC, Ratcliffe A, Rosenwasser MP, et al. Experimental studies on repair of large osteochondral defects at a high weight bearing area of the knee joint: a tissue engineering study. J Biomech Eng. 1991;113:198–207. doi: 10.1115/1.2891235. [DOI] [PubMed] [Google Scholar]
- 17.Hung CT, Mauck RL, Wang CC, et al. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 18.Ng KW, Lima EG, Bian L, et al. Passaged adult chondrocytes can form engineered cartilage with functional mechanical properties: a canine model. Tissue Eng Part A. 2010;16:1041–1051. doi: 10.1089/ten.tea.2009.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bian L, Fong JV, Lima EG, et al. Dynamic mechanical loading enhances functional properties of tissue-engineered cartilage using mature canine chondrocytes. Tissue Eng Part A. 2010;16:1781–1790. doi: 10.1089/ten.tea.2009.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mauck R, Soltz M, Wang C, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng-T ASME. 2000;122:9. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 21.Kelly T-AN, Roach BL, Weidner ZD, et al. Tissue-engineered articular cartilage exhibits tension-compression nonlinearity reminiscent of the native cartilage. J Biomech. 2013;46:1784–1791. doi: 10.1016/j.jbiomech.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffith LG, Naughton G. Tissue engineering-current challenges and expanding opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 23.Bian L, Angione S, Lima E, et al. Tissue-engineered cartilage constructs using mature bovine chondrocytes: effects of temporal exposure to growth factors and dynamic deformational loading. Trans Orthop Res Soc. 2007;32:1480. [Google Scholar]
- 24.Ng KW, O'Conor CJ, Lima EG, et al. Primed mature canine chondrocytes can develop an engineered cartilage tissue with physiologic properties. Trans Orthop Res Soc. 2008;33:599. [Google Scholar]
- 25.Ng KW, O'Conor CJ, Kugler LE, et al. Transient supplementation of anabolic growth factors rapidly stimulates matrix synthesis in engineered cartilage. Ann Biomed Eng. 2011;39:2491–2500. doi: 10.1007/s10439-011-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lima EG, Bian L, Ng KW, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthr Cartilage. 2007;15:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byers BA, Mauck RL, Chiang IE, et al. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14:1821–1834. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 29.McGowan K, Kurtis M, Lottman L, et al. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen® and Hoechst 33258. Osteoarthr Cartilage. 2002;10:580–587. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 30.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 31.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 32.Hollander AP, Heathfield TF, Webber C, et al. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722–1732. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin W, Shuster S, Maibach HI, et al. Patterns of hyaluronan staining are modified by fixation techniques. J Histochem Cytochem. 1997;45:1157–1163. doi: 10.1177/002215549704500813. [DOI] [PubMed] [Google Scholar]
- 34.Pylawka TK, Virdi AS, Cole BJ, et al. Reversal of suppressed metabolism in prolonged cold preserved cartilage. J Orthop Res. 2008;26:247–254. doi: 10.1002/jor.20487. [DOI] [PubMed] [Google Scholar]
- 35.Gregory MH, Capito N, Kuroki K, et al. A review of translational animal models for knee osteoarthritis. Arthritis. 2012;2012:764621. doi: 10.1155/2012/764621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahern BJ, Parvizi J, Boston R, et al. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthr Cartilage. 2009;17:705–713. doi: 10.1016/j.joca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Cook JL, Hung CT, Kuroki K, et al. Animal models of cartilage repair. Bone Joint Res. 2014;3:89–94. doi: 10.1302/2046-3758.34.2000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gortz S, Bugbee WD. Allografts in articular cartilage repair. J Bone Joint Surg Am. 2006;88:1374–1384. doi: 10.2106/00004623-200606000-00030. [DOI] [PubMed] [Google Scholar]
- 39.Lima EG, Chao PHG, Ateshian GA, et al. The effect of devitalized trabecular bone on the formation of osteochondral tissue-engineered constructs. Biomaterials. 2008;29:4292–4299. doi: 10.1016/j.biomaterials.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malinin T, Temple HT, Buck BE. Transplantation of osteochondral allografts after cold storage. J Bone Joint Surg Am. 2006;88A:762–770. doi: 10.2106/JBJS.D.02991. [DOI] [PubMed] [Google Scholar]
- 41.Ranawat AS, Vidal AF, Chen CT, et al. Material properties of fresh cold-stored allografts for osteochondral defects at 1 year. Clin Orthop Relat Res. 2008;466:1826–1836. doi: 10.1007/s11999-008-0311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shasha N, Krywulak S, Backstein D, et al. Long-term follow-up of fresh tibial osteochondral allografts for failed tibial plateau fractures. J Bone Joint Surg Am. 2003;85A:33–39. doi: 10.2106/00004623-200300002-00005. [DOI] [PubMed] [Google Scholar]
- 43.Allen RT, Robertson CM, Pennock AT, et al. Analysis of stored osteochondral allografts at the time of surgical implantation. Am J Sport Med. 2005;33:1479–1484. doi: 10.1177/0363546505275010. [DOI] [PubMed] [Google Scholar]
- 44.Garrity JT, Stoker AM, Sims HJ, et al. Improved osteochondral allograft preservation using serum-free media at body temperature. Am J Sport Med. 2012;40:2542–2548. doi: 10.1177/0363546512458575. [DOI] [PubMed] [Google Scholar]
- 45.Kwan MK, Wayne JS, Woo SLY, et al. Histological and biomechanical assessment of articular-cartilage from stored osteochondral shell allografts. J Orthop Res. 1989;7:637–644. doi: 10.1002/jor.1100070503. [DOI] [PubMed] [Google Scholar]
- 46.Pallante AL, Bae WC, Chen AC, et al. Chondrocyte viability is higher after prolonged storage at degrees C than at 4 degrees C for osteochondral grafts. Am J Sport Med. 2009;37:24s–32s. doi: 10.1177/0363546509351496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rohde RS, Studer RK, Chu CR. Mini-pig fresh osteochondral allografts deteriorate after 1 week of cold storage. Clin Orthop Relat R. 2004;427:226–233. doi: 10.1097/01.blo.0000138955.27186.8e. [DOI] [PubMed] [Google Scholar]
- 48.Williams SK, Amiel D, Ball ST, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am 85-A. 2003:2111–2120. doi: 10.2106/00004623-200311000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Williams JM, Virdi AS, Pylawka TK, et al. Prolonged-fresh preservation of intact whole canine femoral condyles for the potential use as osteochondral allografts. J Orthop Res. 2005;23:831–837. doi: 10.1016/j.orthres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Hansbrough JF, Mozingo DW, Kealey GP, et al. Clinical trials of a biosynthetic temporary skin replacement, dermagraft-transitional covering, compared with cryopre-served human cadaver skin for temporary coverage of excised burn wounds. J Burn Care Rehabil. 1997;18:43–51. doi: 10.1097/00004630-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Lanza RP, Langer RS, Vacanti J. Principles of tissue engineering. 4th ed. Academic Press an imprint of Elsevier; Amsterdam: 2014. p. 1887. [Google Scholar]
- 52.Hart CE, Loewen-Rodriguez A, Lessem J. Dermagraft: use in the treatment of chronic wounds. Adv Wound Care. 2012;1:138–141. doi: 10.1089/wound.2011.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muhart M, McFalls S, Kirsner RS, et al. Behavior of tissue-engineered skin: a comparison of a living skin equivalent, autograft, and occlusive dressing in human donor sites. Arch Dermatol. 1999;135:913–918. doi: 10.1001/archderm.135.8.913. [DOI] [PubMed] [Google Scholar]