Figure 3.
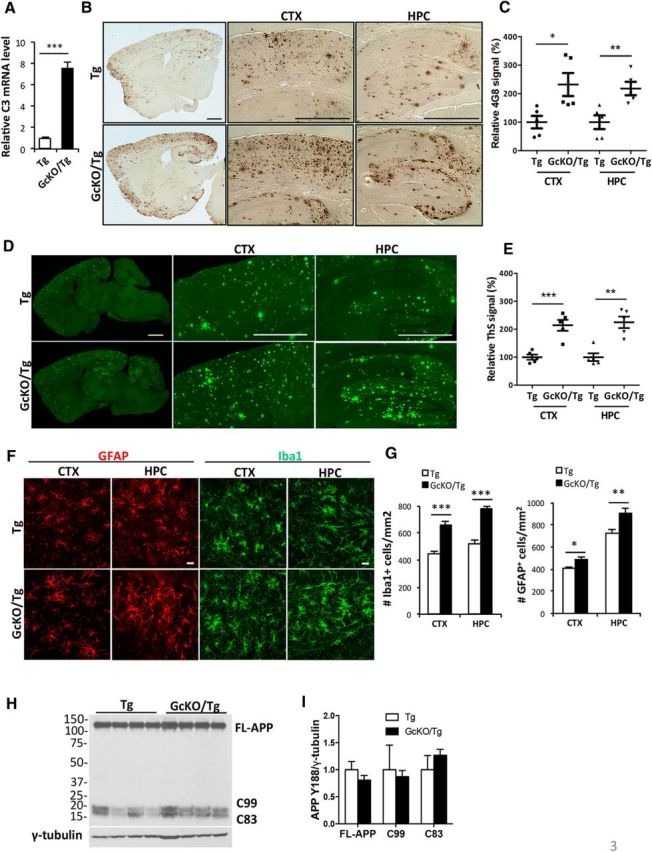
Astrocyte-specific NF-κB/C3 activation increases amyloid burden and exacerbates reactive gliosis in APP/PS1 transgenic (Tg) mice. A, qPCR measurement of C3 mRNA levels in Tg animals with astrocyte-specific IκBα deletion and NF-κB/C3 activation (GcKO/Tg) and Tg mice alone. N = 3 animals per genotype. B, Representative DAB immunostaining of amyloid plaques in Tg and GcKO/Tg animals using the rabbit anti-Aβ antibody. CTX, Cortex; HPC, hippocampus. C, Quantification of plaque load in the CTX and HPC of Tg and GcKO/Tg animals. N = 5 animals per genotype, 4–5 sections per mouse. D, Representative Thioflavin S (ThS) staining of amyloid plaques in the CTX and HPC of Tg and GcKO/Tg animals. E, Quantification of ThS-positive signals in the CTX and HPC of Tg and GcKO/Tg animals. N = 5 animals per genotype, 4–5 sections per mouse. F, Representative GFAP and Iba1 immunostaining in CTX and HPC of Tg and GcKO/Tg animals. G, Quantification of GFAP+ and Iba1+ cells in the CTX and HPC. N = 16 sections per genotype (GFAP), N = 13 sections per genotype (Iba1). H, I, Western blot analysis of FL-APP and its C-terminal fragments C83 and C99 using the APP Y188 antibody in Tg and GcKO/Tg animals (H) and its quantification (I, nonsignificant). Scale bars: B, D, 1 mm; F, 20 μm. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 (two-tailed Student's t test). Filled circle, Tg CTX; filled square: GcKO/Tg CTX; filled triangle, Tg HPC; filled reverse triangle, GcKO/Tg HPC.
