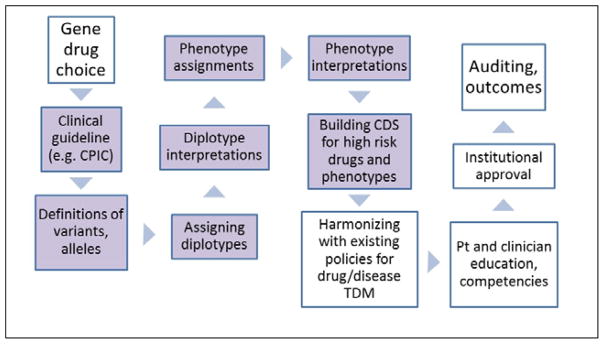
Figure 3.
Multiple steps in bringing pharmacogenomic tests to the clinic. These include prioritizing the choice of gene(s)/drug(s) for actionability; CPIC guidelines exist or are being developed for all actionable inherited pharmacogenes,24 and the guidelines provide guidance for the steps shaded in lavender. Genotypes must be assigned to alleles, and diplotypes assigned to patients. The diplotypes must be translated into phenotypes (gene function) and interpreted with respect to drug therapy. Appropriate clinical decision support (CDS) should be built and deployed to provide prescribers with recommendations, and pharmacogenetic considerations must be harmonized with other policies for the affected medications, using therapeutic drug monitoring (TDM) where applicable. Education of clinicians and of patients should take place, and institutional oversight committees may approve prescribing recommendations and policies. Many groups are auditing clinical and prescribing outcomes to evaluate the impact of clinical implementation of pharmacogenomics.