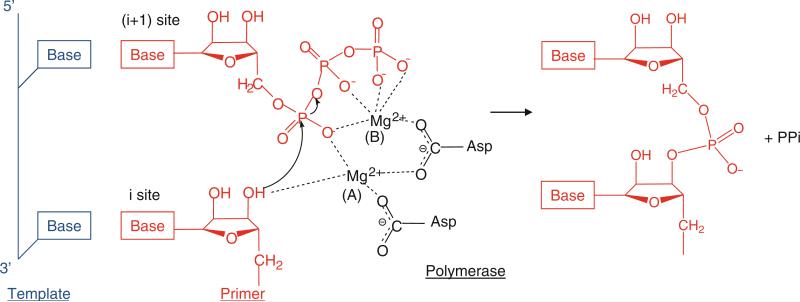
Fig. 12.2.
Two-metal mechanism used by polymerases to catalyze the nucleotidyl transfer reaction. Template, primer, and polymerase residues are shown in blue, red, and black, respectively. The polymerase active site contains the binding sites for a template, the RNA or DNA terminus (the initiation, “i”, or priming site), and the incoming NTP (the “i + 1” site). Two metal ions, A and B, are coordinated by the conserved Asp residues in the polymerase active site. Metal ion A binds to the 3′-OH of the primer terminus and lowers the affinity of the 3′-OH for its hydrogen, facilitating a nucleophilic attack of 3′-O− on the α-phosphate of the incoming NTP (Steitz 1998). Metal ion B stabilizes the incoming NTP and pyrophosphate (ppi) leaving group