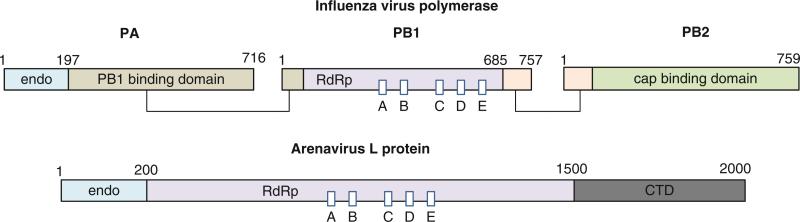
Fig. 12.9.
Domain arrangement of RNA-dependent RNA polymerases from negative-sense ssRNA viruses. Influenza virus polymerase consists of three subunits, PA, PB1, and PB2 (top). All RNA polymerase motifs are found in PB1. The N-terminal domain of PA has an endonuclease (endo) activity that is proposed to be involved in the cap-snatching mechanism. Domains known to interact with each other are shown in the same color and are connected with lines (Boivin et al. 2010). Arenavirus L protein consists of a single polypeptide chain of 2,000 amino acids (bottom). Endonuclease (endo), RNA-dependent RNA polymerase (RdRp), and C-terminal domains (CTD) are labeled. Sequence motifs common to all RdRps (boxed and labeled A–E) are shown with the active site Ser-Asp-Asp (GDD) motif located in box C