Table 2.
Enantioselective carboalkoxylation reactions.[a]
|
| |||||
|---|---|---|---|---|---|
| entry | R | R1–X | product | yield (%)[b] | er |
| 1c | (CH2)4 (1a) |
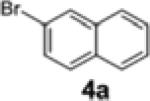
|
2a | 62 | 88:12 |
| 2 | 1a | 4a | 2a | 58 | 89:11 |
| 3[c] | 1a |
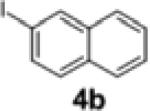
|
2a | 59 | 87:13 |
| 4 | 1a |
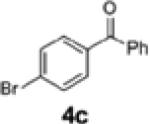
|
2b | 54 | 82:18 |
| 5 | H (1b) | 4c | 2c | 23 | 58:42 |
| 6[e] | Ph (1c) |
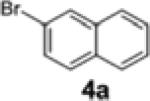
|
2d | 67 | 95:5 |
| 7[c,d,e] | 1c | 4a | 2d | 60 | 96:4 |
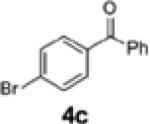
| 8 | 1c |
|
2e | 64 | 92:8 |
| 9c,d | 1c | 4c | 2e | 61 | 95:5 |
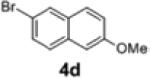
| 10 | 1c |
|
2f | 66 | 95:5 |
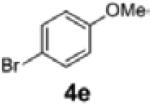
| 11 | 1c |
|
2g | 63 | 94:6 |
| 12[c,d] | 1c | 4e | 2g | 54 | 95:5 |
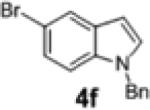
| 13 | 1c |
|
2h | 71 | 93:7 |
| 14 | 1c |
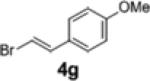
|
2i | 18 | 79:21 |
Conditions: 1.0 equiv substrate, 1.8 equiv R1–X, 1.8 equiv NaOtBu, 2 mol % Pd2(dba)3, 5 mol % L7, dioxane (0.2 M), 90 °C, 12-14 h. Reactions were conducted on a 0.20 mmol scale. Small amounts (ca 10–15%) of regioismeric products analogous to 3a were also obtained in reactions of substrates 1a and 1b. Product 2a could be easily separated from the regioisomer, whereas the regioisomer could not be separated from 2b and the yield is for the mixture of products.
Isolated yield (average of two or more runs).
The reaction was conducted in toluene solvent.
2 equiv of H2O was added to the reaction mixture.
The reaction was conducted using 1.4 equiv Ar–X.
