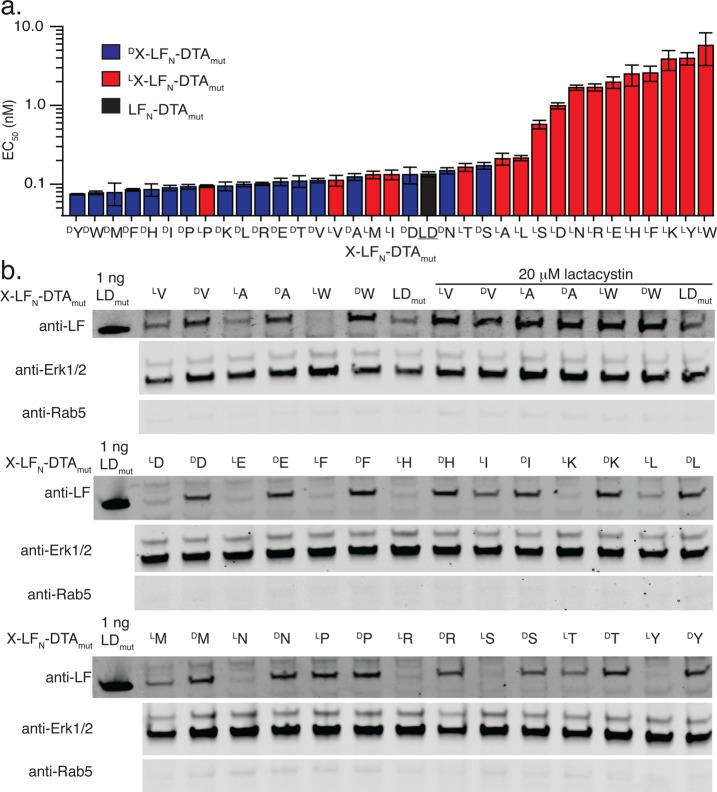
Figure 2.
One N-terminal d-amino acid on LFN-DTA enhances protein stability. (a) Translocation X-LFN-DTA constructs was analyzed by protein synthesis inhibition assay in CHO-K1 cells after 6 h (n = 3). EC50 values from the protein synthesis inhibition assay were graphed for all LX-LFN-DTA or DX-LFN-DTA constructs. EC50 values (and error bars) were determined using a Boltzmann distribution fit. (b) LV-, DV-, LA-, DA-, LW-, and DW-LFN-DTAmut were translocated into CHO-K1 cells in the presence of 20 nM PA for 6 h, then extracted using digitonin lysis buffer, and analyzed by Western blot. As a proteasomal inhibitor, 20 μM lactacystin was used. Translocation of all LX-LFN-DTA or DX-LFN-DTA constructs was analyzed by Western blot.