Figure 4.
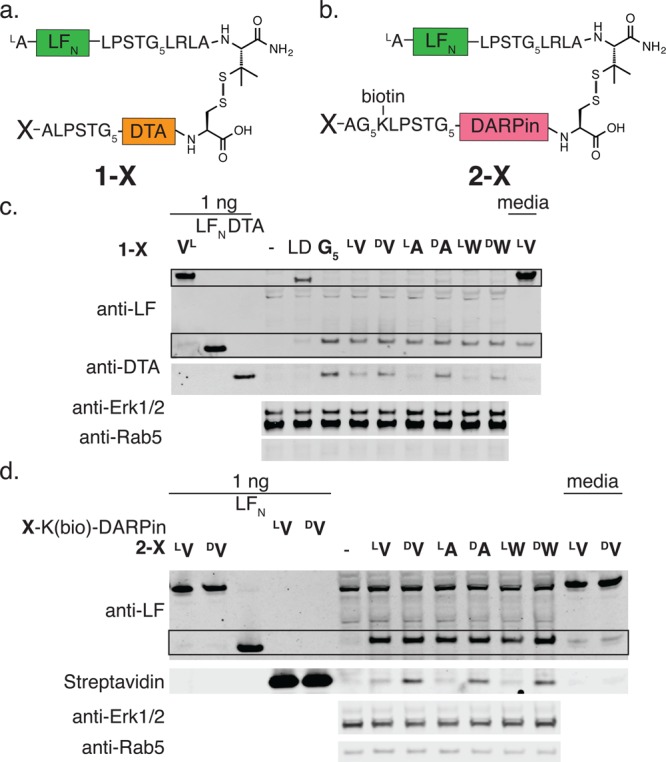
N-terminal d-amino acid stabilization is not limited to LFN. (a) Molecular composition of X-DTAmut conjugated to LFN through a hindered disulfide (1-X), where X represents G5, LV, DV, LA, DA, LW, or DW. (b) Molecular composition of X-DARPin conjugated to LFN through a hindered disulfide (2-X), where X represents LV, DV, LA, DA, LW, or DW. (c) CHO-K1 cells were treated with 100 nM 1-X conjugates in the presence of 20 nM PA for 6 h, then extracted using digitonin lysis buffer, and analyzed by Western blot. The absence of full-length material suggests that each construct was appropriately reduced in the cytosol. Furthermore, LFN (LA as the native N-terminus) and X-DTAmut bands indicated cleavage and stabilization of the X-DTAmut cargo with one N-terminal d-amino acid. The postincubation medium was analyzed by Western blot to indicate the stability of the hindered disulfide over the time of the experiment. (d) CHO-K1 cells were treated with 100 nM 2-X conjugates in the presence of 20 nM PA for 6 h, then extracted using digitonin lysis buffer, and analyzed by Western blot using anti-LF and streptavidin staining. LFN and X-DARPin bands indicated cleavage and stabilization of the X-DARPin cargo with one N-terminal d-amino acid.
