Abstract
Mixture of pomegranate juice and whey was evaluated as a potential substrate for production of a novel beverage by kefir grains. The effects of two different variables, fermentation, temperature (19 and 25 °C) and kefir grain amount (5 %w/v and 8 %w/v), on total phenolic content (TPC) and antioxidant activities of beverage were examined during a fermentation time of 32 h. TPC and antioxidant activities including 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging, reducing power, inhibition effect upon linoleic acid autoxidation and inhibition effect upon ascorbate autoxidation increased significantly (p < 0.05) during fermentation, but metal chelating effect showed no significant difference. The highest increases were observed when the temperature of 25 °C and kefir grain amount of 8 %w/v were applied. Results proved antioxidant activities of beverages were desirable and fermentation by kefir grains has the ability to enhance these antioxidant activities, as compared with unfermented beverage. Also pomegranate juice and whey were suitable media for producing a novel dairy-juice beverage.
Keywords: Pomegranate juice, Whey, Kefir grain, Fermentation, Total phenolic content, Antioxidant activity
Introduction
Kefir which is made with kefir grains is a refreshing, naturally carbonated fermented dairy beverage that has a slightly acidic taste, yeasty flavor and creamy consistency. The traditional kefir is produced by the addition of small kefir grains to fresh milk. Kefir grains are like small cauliflower florets, 1–3 cm in length, lobed, irregularly shaped, white to yellow-white in color, and have a slimy but firm texture (La Riviére et al. 1967). Lactic acid bacteria (LAB) and yeasts have a complex symbiotic relationship in kefir grains and are responsible for lactic acid and alcoholic fermentation, respectively. Since microorganisms in kefir grains are able to metabolize lactose, they can be used to ferment cheese whey which is a lactose-rich waste of negligible cost. Whey is a by-product of the cheese-making industry, contains about 85–95 % of the milk volume and is rich in nutrients such as lactose, soluble proteins, lipids, minerals, vitamins, and organic acids. Whey proteins include α-lactalbumin (α-La), β-lactoglobulin (β-Lg), bovine serum albumin and immunoglubolins. β-Lg is a small and soluble globular protein which has a lot of useful nutritional and functional food characteristics. Also this protein shows many biological activities such as anti-hypertensive, anti-cancer, hypo-cholesterolemic and anti-microbial (Chatterton et al. 2006). α-La is another major protein in whey that makes up about of 25 % of total whey proteins. α-La is a calcium binding protein and enhances calcium absorption. Amino acids such as lysine, leucine, threonine, tryptophan and cysteine are present in large amounts in the whey (Permyakov and Berliner 2000). The antioxidant activity of antioxidant components have been attributed to various mechanisms. To best of our knowledge, among of all antioxidant properties, radical scavenging, reducing power, metal chelating effect, total antioxidant activity, inhibition effect upon linoleic acid autoxidation and inhibition effect upon ascorbate autoxidation are most commonly used for the evaluation of antioxidant activity (Liu et al. 2005; Wang et al. 2006; Marazza et al. 2012; Sun et al. 2009). Some oxidative damages in food systems and human body are caused by several free reactive radicals such as superoxide radical, hydroxyl radical and peroxide radical, which are generated by exogenous or endogenous metabolic processes. These free radicals can result in diseases including cancer, atherosclerosis, hypertension, arthritis, emphysema and cirrhosis (Kehrer 1993). Iron is a metal with high reactivity and is known as the most important lipid pro-oxidant. In pathological conditions, iron can work as a catalyst for generating of reactive oxygen species. Some of diseases like cancer and cardiovascular can be a result of metal ion catalysis (Liu et al. 2005). Hydrogen and lipid peroxides break down by the ferrous form of iron and produce reactive free radicals during Fenton reaction. Mono and polyunsaturated fatty acids produced during food processing and storage, are major concern for food industry. Lipid peroxides are toxic and most body cells in the human body are damaged by them (Liu et al. 2005). In the process of lipid peroxidation first a fatty acid or fatty acyl side chain is attacked by any chemical species that features sufficient reactivity to abstract a hydrogen atom from a methylene carbon in the side chain. Lipid radicals resulted from the reaction then undergo molecular rearrangement, react with oxygen and produce peroxyl radicals. These radicals are capable to abstract hydrogen from adjacent fatty acid side chains and propagate a chain reaction of lipid peroxidation (Halliwell and Gutteridge 1984). Hence inhibition of lipid peroxidation has great important for prevention of the food quality deterioration and human diseases relating to free radicals.
However there is an inherently anti oxidative system including superoxide dismutase, glutathione peroxidase and uric acid in our body that can protect us from some damages generated by reactive oxygen species (ROS), but this system is not enough for protecting against all of these damages (Simic 1988). Many kinds of fruits and vegetables are known as natural antioxidant sources which are more desirable than chemical antioxidant. Red fruit juices have got great attention because of their phenolic content and antioxidant activities. Phenolic compounds have many beneficial effects on human bodies and can inhibit some of diseases such as cancer. Pomegranate (Punica granatum L.) is one of the oldest edible fruits and contain many kinds of polyphenol components such as punicalagin isomers, ellagic acid derivatives and anthocyanins (delphinidin, cyanidin, pelargonidin 3- and 3,5-diglucosides) (Pokorny and Schmidt 2003). The antioxidant activities of pomegranate juice have been studied earlier (Gil et al. 2000; Zaouay et al. 2012; Fawole et al. 2011). Because of polyphenol existence in fruits and vegetables that have redox properties, fruits and vegetables can act as reducing agent, hydrogen donor and singlet oxygen quencher (Brand-Williams et al. 1995). Also proteins from several animal sources can act as antioxidant. Milk protein hydrolysates and individual peptides released after hydrolysis expressed antioxidant activity against some oxidative components. For instance certain amino acid sequences such as histidin and some hydrophobic amino acids are known as natural antioxidant sources (Suetsuna et al. 2000).
The aim of this study is to determine the TPC and antioxidant activities of a novel fermented beverage based on pomegranate juice and whey using kefir grains as starter culture. Also changes of total phenolic content and antioxidant activity during fermentation will be examined. In previous study (Sabokbar and Khodaiyan 2014) apple juice was used and good results were obtained for sensory evaluation and antioxidant activities. Therefore in present study pomegranate will be used because first examined showed mixture of pomegranate juice and whey would be have good sensory properties such as color and taste.
Materials and methods
Kefir grains were collected from a household in Tehran, Iran. The grains were kept in pasteurized milk at room temperature. Milk was exchanged every 2 days to maintain the grains viability. The commercial concentrated pomegranate juice and whey used in this study were supplied from Alifard (Sunich, Iran, Saveh) and Safadasht (Iran, Karaj) cheese making company, respectively.
Preparation of fermented beverage
Whey was diluted with distilled water in a portion of 1:1 and then mixed with pomegranate juice concentrate to 14° Brix. Mixture was pasteurized at 60 °C for 30 min. Kefir grains were removed from milk, washed with distilled water and then inoculated into the prepared beverage at two levels (5 % and 8 % w/v). The beverage was incubated at two temperatures (19 and 25 °C) for 32 h. The fermentation runs were assessed through periodic sampling in order to determine characteristics of beverages. After completion of fermentation the kefir grains were removed, washed with distilled water and returned to milk and beverage was prepared for further analysis. Control sample (unfermented beverage) contained the same proportion of whey and concentrated pomegranate juice, but the fermentation process by kefir grains did not apply for it.
Measurement of total phenolic content
The TPC of each sample was determined according to Folin–Ciocalteau method (Singleton and Rossi 1965). Briefly 0.2 mL of diluted beverage was added to 1 mL of Folin–Ciocalteau reagent (prediluted 10-fold with distilled water) and shaken well. Mixture was allowed to stand at room temperature for 8 min. Then 0.8 mL of sodium carbonate (7.5 %) was added to mixture, shaken and left at room temperature for 30 min. Absorbance was measured at 765 nm in a spectrophotometer (CECIL CE 250). The TPC was assessed by plotting the gallic acid calibration curve and expressed as milligrams of gallic acid equivalents per liter of sample. Eight different dilutions (in the range of 0.22 mg GA/L-80 mg GA/L) were used to get the gallic acid calibration curve. The equation for the gallic acid calibration curve was Y = 89.014X – 34.479 (where X = measured absorbance and Y = concentration of gallic acid equivalents expressed as milligrams of GA per liter of sample), and the correlation coefficient was R2 = 0.9751.
Antioxidant capacity determination
Scavenging effect upon 1, 1-Diphenyl-2-picrylhydrazyl (DPPH) radicals
The free radical scavenging activity of beverages was measured by DPPH● using the method of Brand-Williams et al (1995). Three dilutions of each beverage were prepared. Then 3.9 mL of a 25 mg/L methanolic solution of DPPH● was added to 0.1 mL of each diluted samples. Mixtures were shaken well. The control sample was prepared with the same volume of methanol instead of beverage. Mixtures were left at room temperature (in a dark place) for 30 min and after that absorbance were measured at 515 nm using a CECIL CE 250 spectrophotometer. The DPPH● concentration in the reaction mixture was calculated using equation y = 35.919A515–1.9031 (R2 = 0.9971) as was obtained by liner regression containing different concentration of DPPH●. The % of remaining DPPH was calculated as follow:
Where [DPPH●]t and [DPPH●]t=0 were the DPPH● concentration of reaction mixture after 30 min (steady state) and DPPH● concentration of control, respectively. The %remaining of DPPH● was plotted against the beverage concentration to obtain EC50. EC50 is the concentration of beverage which can decrease the initial DPPH● concentration by 50 %. Lower EC50 value shows higher radical scavenging activity.
Ferrous ion chelating ability
The ferrous ion chelating ability of beverages was measured according to the method of Decker and Welch (1990). Briefly 5 mL of beverage was mixed with 0.1 mL of ferrous chloride (2 mM) and 0.2 mL of ferrozine (5 mM). The mixture was shaken and allowed to stand at room temperature for 10 min. Then absorbance was measured at 562 nm using a spectrophotometer (CEILE CE 2502, 2000 series, England). Chelating effect of samples was calculated as follow:
Where the A0 is the absorbance of control and A1 is the absorbance in the presence of the beverage. The control contains FeCl2 and ferrozine, with no beverage.
Reducing power
The reducing power of beverages was measured according to the Oyaizu’s method (1986). 2.5 mL of each beverage was mixed with 2.5 mL of sodium phosphate buffer (0.2 M, pH = 6.6) and 2.5 mL of potassium ferricyanide (1 %). The mixture was incubated at 50 °C for 20 min. Then 2.5 mL of thrichloro acetic acid (1 %) was added to the mixture and mixture was centrifuged at 1400 g for 10 min. The upper layer of solution (5 mL) was mixed with 5 mL of distilled water and 1 mL of ferric chloride (0.1 %). The absorbance of the mixture was measured at 700 nm in a spectrophotometer (CECIL CE 250, England). Greater absorbance shows greater reducing power.
Inhibition effect upon lipid peroxidation
The inhibition effect of beverage upon lipid peroxidation was determined according to Yen et al. (2000). The linoleic acid emulsion was prepared by mixing equal volumes of linoleic acid, Tween 20, and phosphate buffer (0.02 M at pH 7.0). Then samples (0.5 mL) were mixed with 2.5 mL of linoleic acid emulsion (0.002 M) and 2 mL of phosphate buffer (0.2 M at pH 7). The reaction mixture was incubated at 50 °C in the dark, and the degree of oxidation was measured according to the thiocyanate method (Yen et al. 2000). Ethanol (4.7 mL, 75 %), 0.1 mL of ammonium thiocyanate (30 %), 0.1 mL of sample solution, and 0.1 mL of ferrous chloride (20 mM) were added sequential in HCl (3.5 %). Then mixture was stirred for 3 min and peroxide value was determined by reading the absorbance at 500 nm. The relative inhibition of linoleic acid peroxidation was calculated as below:
Where the A0 is the absorbance of control and A1 is the absorbance in the presence of the beverage.
Inhibition effect upon ascorbate autoxidation
The method of Mishra and Kovachich (1984) was used to determine the inhibition of ascorbate autoxidation. 0.1 mL of sample or distilled water (control) was mixed with an ascorbate solution (0.1 mL, 5.0 mM) and phosphate buffer (9.8 mL, 0.2 M, pH 7.0). Mixture was placed at 37 °C for 10 min and then the absorbance of this mixture was measured at 265 nm. The ascorbate autoxidation inhibition rate of the sample was calculated according to the following equation:
A0 is the absorbance of control and A1 is the absorbance of sample.
Statistical analysis
All experiments were carried out in triplicate. Statistics on a completely randomized design were performed with the analysis of variance (ANOVA) procedure. Duncan’s multiple range tests were used to compare the difference among mean values of beverage’s properties at the level of p = 0.05. SAS software (version 9.1; statistical analysis system institute Inc., Cary, NC, USA) was used for analysis.
Results and discussion
Total phenolic content
As shown in Table 1, total phenolic contents in the examined beverages were the highest in beverage fermented with 8 %w/v kefir grains at temperature of 25 °C, 249 ± 7.01 mg GA/L. Lower total phenolic contents were present in beverage fermented at temperature of 19 °C with 8 %w/v kefir grains and 25 °C with 5 %w/v kefir grains, and the lowest in fermented beverage with 5 %w/v kefir grains at temperature of 19 °C. Fermentation affected the TPC as shown in Table 1. On the other hand it can be seen from Table 1 that type of fermentation could affect the total phenolic content changes. In beverages fermented with different levels of kefir grains but in same temperature, with increase in kefir grain level from 5 % w/v to 8 % w/v increase in total phenolic contents was significantly (P < 0.05) higher. Also temperature of 25 °C was more effective on TPC increase in beverages fermented with the same level of kefir grains. This might be duo to the optimum temperature for enzymes or metabolic activities of microorganisms in kefir grains. Also, it was observed that different fermentation conditions resulted in difference at pH (Sabokbar and Khodaiyan 2014). Therefore, this difference is another reason for these results, knowing that optimum pH influences the liberation of enzymes derived from the microorganisms (Boskov-Hansen et al. 2002).
Table 1.
TPC and antioxidant activities of various fermented beverages and control sample
| Sample | TPC (mgGA/l) |
EC50
(mL/mL) |
Reducing power (absorbance at 700 nm) |
Chelating effect (%) | Inhibition effect upon linoleic acid autoxidation (%) | Inhibition effect upon ascorbate autoxidation (%) | |
|---|---|---|---|---|---|---|---|
| Fermentation temperature (°C) | Kefir grains inoculation (%w/v) | ||||||
| 19 | 5 | 191 ± 6.21c | 0.43 ± 0.03b | 0.660 ± 0.038c | 51.00 ± 2.00a | 42.32 ± 2.00d | 17.90 ± 0.79c |
| 19 | 8 | 216 ± 6.52b | 0.34 ± 0.02c | 0.802 ± 0.030b | 50.90 ± 2.01a | 56.90 ± 1.85b | 24.03 ± 0.91a |
| 25 | 5 | 214 ± 4.11b | 0.35 ± 0.02c | 0.790 ± 0.035b | 50.37 ± 1.80a | 49.98 ± 1.800c | 20.21 ± 0.85b |
| 25 | 8 | 249 ± 7.01a | 0.27 ± 0.03d | 0.951 ± 0.032a | 50.80 ± 2.02a | 64.09 ± 2.00a | 25.34 ± 1.00a |
| Control (unfermented) | 101 ± 5.03d | 0.76 ± 0.03a | 0.100 ± 0.029d | 50.00 ± 1.92a | 20.03 ± 1.50e | 10.20 ± 0.65d | |
Means within the same column with different letters are significantly (P < 0.05) different. Data are means ± standard deviation
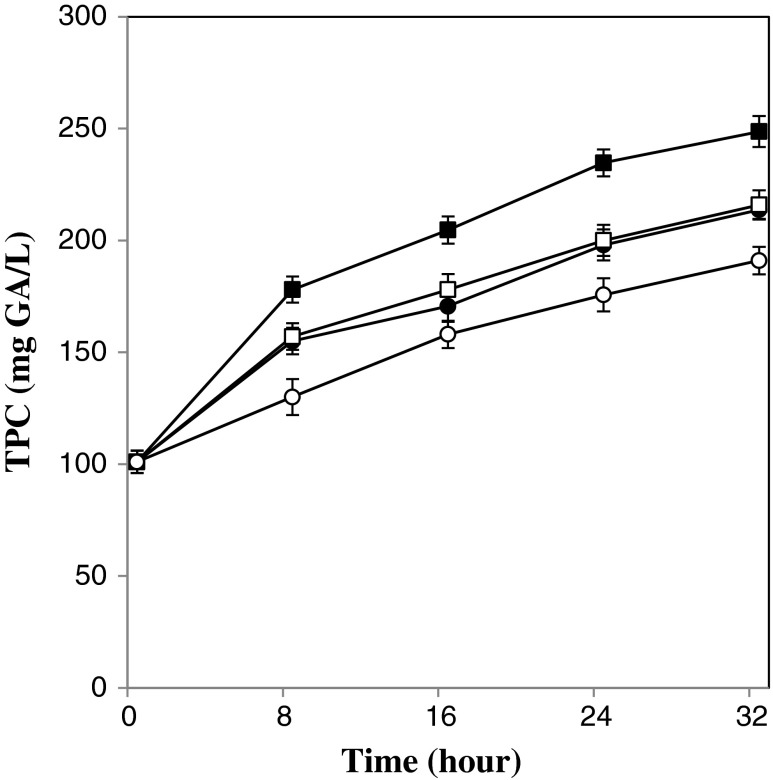
In all fermented beverages TPC increased significantly (p < 0.05) from initial amount of 101 ± 5 mg GA/L for control (unfermented) beverage to higher level, depending on type of fermentation. Also Fig. 1 shows that changes in TPC of different fermented samples during 32 h of fermentation. It can be seen TPC enhanced as the fermentation period extended. These results can be explained by the fact that the metabolic activities of microorganisms in kefir grains can modify the levels of bioactive components such as different phenolic compounds. During fermentation enzymes such as B-glycosidase derived from the fermentative microorganisms are responsible for hydrolyzing of complex phenolic compounds to simpler types and increase in quantitative amount of TPC (Mousavi et al. 2011). Other enzymes such as protease derived from the microorganisms or whey can have contributed to the modification of beverage compositions. It has been reported by other researchers that fermentation by lactic acid bacteria or other microorganisms can increase the level of total phenolic content, too (Dordević et al. 2010; Voung et al. 2006; Coda et al. 2012).
Fig. 1.
Changes in TPC of beverages during the fermentation process. Fermentation temperature of 19 °C: open symbol, Fermentation temperature of 25 °C: closed symbol, kefir inoculation of 5 % w/v: circle, kefir inoculation of 8 % w/v: square. Bars represent the standard deviation
Antioxidant capacity
DPPH radical scavenging
DPPH radical scavenging (was expressed as EC50) of different beverages are shown in Table 1. As can be observed the highest DPPH radical scavenging ability was found in beverage fermented with 8 % w/v kefir grains at temperature of 25 °C since EC50 value for this beverage was 0.27 ± 0.03 mL/mL. The lower DPPH radical scavenging was observed for fermented beverages with 8 % w/v kefir grains at temperature of 19 °C and 5 % w/v kefir grains at 25 °C. While the highest value for EC50 and hence the lowest DPPH radical scavenging among fermented beverages was found in beverage fermented with 5 % w/v kefir grains at temperature of 19 °C with EC50 value of 0.43 ± 0.03 mL/mL. DPPH radical scavenging activity depended on type of fermentation (Table 1). In beverages fermented at different temperature but with the same level of kefir grains, with increase in temperature from 19 °C to 25 °C DPPH radical scavenging increased significantly (p < 0.05). This might be duo to the optimum temperature for metabolic activities of microorganisms in kefir grains which can have effect on antioxidant activities. About beverages fermented in the same temperature increase in the level of kefir grains inoculation led to increase in DPPH radical scavenging. In this situation it can be said transferring of antioxidant compound (which was cited before) at the higher level of inoculation was higher.
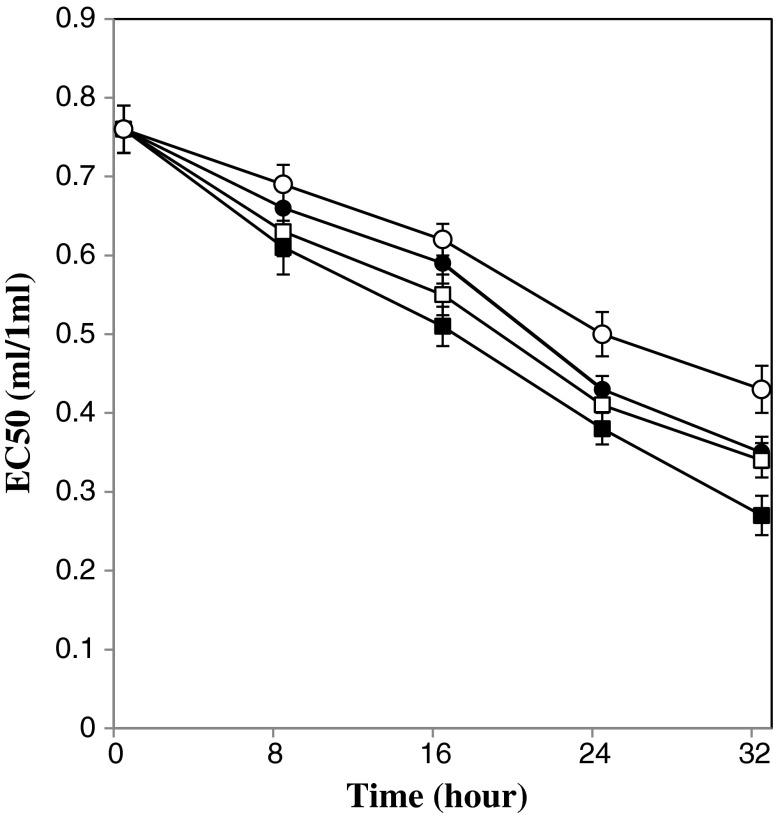
The changes in EC50 value during different cultivation of kefir grains in substrate are depicted in Fig. 2. It was noted regardless of level of kefir grains and fermentation temperature, the ability of fermented beverages to scavenge DPPH radicals increased as the fermentation period extended.
Fig. 2.
Changes in EC50 value of beverages during the fermentation process. Fermentation temperature of 19 °C: open symbol, Fermentation temperature of 25 °C: closed symbol, kefir inoculation of 5 % w/v: circle, kefir inoculation of 8 % w/v: square. Bars represent the standard deviation
Possible antioxidant activities of milk whey have been suggested. Chelatation of transition metals by serum albumin and lactoferrin, an iron-binding glycoprotein, and free radical scavenging activity by amino acids such as tyrosine and cysteine are some of these antioxidant activities (Chatterton et al. 2006). Also, antioxidant activity for some peptide chains in whey such as Trp-Tyr-Ser-Leu-Ala-Met-Ala-Ala-Ser-Asp-Ile was reported (Hernández-Ledesma et al. 2005). This peptide had higher radical scavenging activity as compared to butylated hydroxianisole (BHA). Another compound of beverage prepared in this study which can have antioxidant activity is phenolic compounds, since antioxidant activities (DPPH radical scavenging) of phenolic compounds in fruits have been reported (Lu and Foo 2000). Fermentation with kefir grains had a positive influence on DPPH radical scavenging of beverage (Table 1). EC50 value decreased significantly (p < 0.05) from initial amount of 0.76 ± 0.03 mL/mL in control (unfermented) beverage to different values in fermented beverages depending on fermentation conditions. Also, it has been reported by Marazza et al. (2012) that fermentation of soymilk by L. rhamnosus can increase the DPPH radical scavenging. Similar results have been reported by other researchers (Liu et al. 2005; Voung et al. 2006; Dordević et al. 2010). During fermentation antioxidant compounds in kefir grains are transferred to beverage and lead to increase in DPPH radical scavenging activity (Liu et al. 2005). Also, both intact cells and intracellular cell free extracts of L.acidophilus have ability of DPPH radical scavenging (Lin and Chang 2000) and L.acidophilus is one of the bacteria found in kefir grains. Fermentation can release amino acids such as cysteine in peptide chains of whey protein. Cysteine is able to donate hydrogen atom to DPPH radicals and therefore can neutralize this radical from the purple color to yellow color of non-radical form (diphenyl-picrylhydrazine). Besides, synergistic effect of phenolic compounds with each other or other compounds can have positive effect on antioxidant activity increase such as DPPH radical scavenging (Shahidi et al. 1994). Polyphenols in pomegranate (Gil et al. 2000) and other fruits showed good antioxidant activities (Khanizadeh et al. 2008). As regards to results it can be said increase in TPC after fermentation with kefir grains have positive effect on DPPH radical scavenging activity, since both of them increased after fermentation.
Metal chelating effect
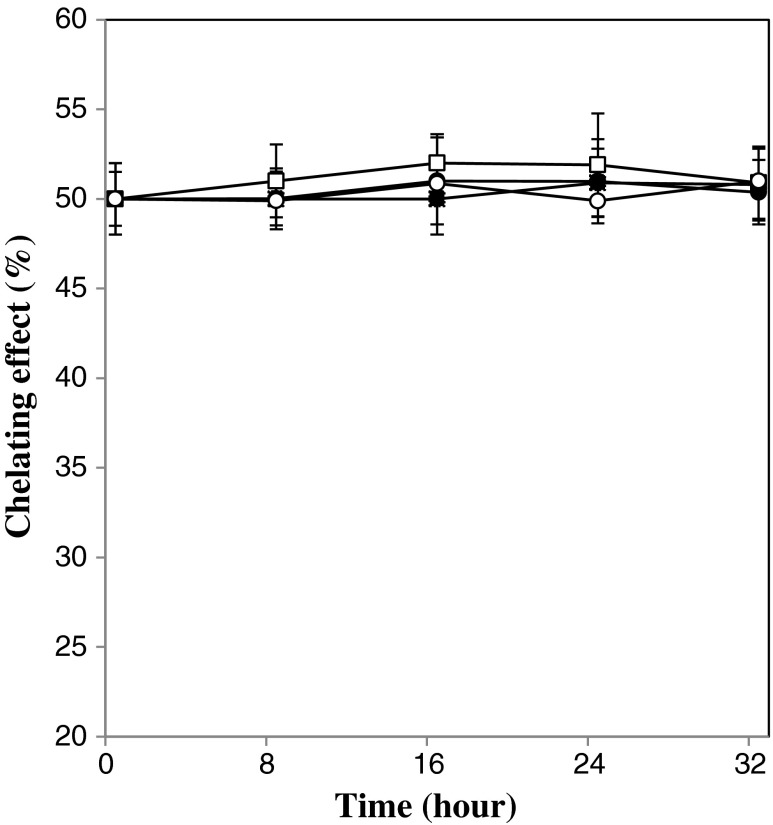
The ferrous ion chelating activities of unfermented and fermented beverages are shown in Table 1. It can be seen that the beverage fermented with different levels of kefir grains in different temperature did not reveal a significant (p < 0.05) increase in their ferrous ion chelating activity as compared to control. Figure 3 shows the changes of ion chelating activities during fermentation period. The chelating effect of present drink can have attributed to whey proteins since the ability of lactoferrin, serum albumin, casein, and a high molecular-weight fraction of whey to chelate ferrous ion have investigated by several researchers (Tong et al. 2000). Milk fractions which have high greater number of phosphoseryl serine group show greater affinity for iron. Although carboxyl group of the amino acids asparagine and glutamine have good ability for binding iron (Wong and Kitts 2003). Chelating effect of beverage also can be attributed to phenolic compounds and pomegranate contains different phenolic compounds. Phenolic compounds derived from soybean, were able to chelate ferrous ion and make a safe and catalytically inactive form (Moran et al. 1997). Besides of goodish chelating effect properties gotten for beverage, but It was observed chelating effect were constant during fermentation. These results are according to Liu et al. (2005) that reported ferrous chelating activity of milk and soy milk did not change by fermentation with kefir grains. Fermentation may alter the composition, structure and polarity of antioxidant biofactors in fermented beverage. Therefore fermentation may be releases some Fe2+-binding factors, breaks some of them or changes the position of the others, for example the phosphoseryl residue located on the surface of casein micelle which according to Wong and Kitts (2003) can have Fe2+-binding effect . To the best of our knowledge only a few previous studies focused on this issue. Nevertheless, the variation of these Fe2+-binding factors present in pomegranate juice, kefir grains and whey and duo to fermentation remained to be further investigated.
Fig. 3.
Changes in chelating effect of beverages during the fermentation process. Fermentation temperature of 19 °C: open symbol, Fermentation temperature of 25 °C: closed symbol, kefir inoculation of 5 % w/v: circle, kefir inoculation of 8 % w/v: square. Bars represent the standard deviation
Reducing power
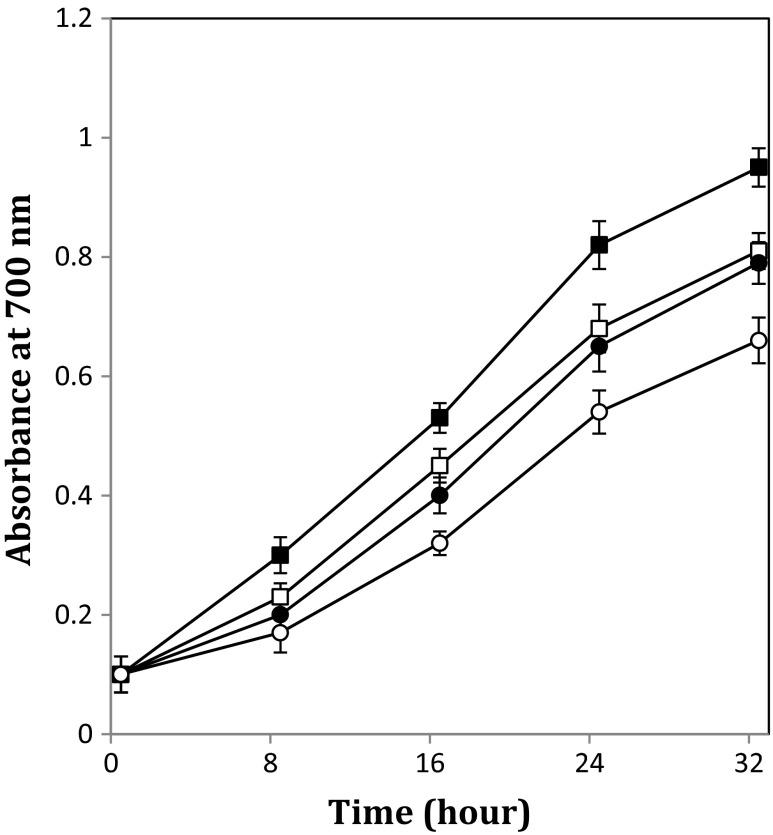
Reducing power of different beverages is shown in Table 1. Beverage fermented with 8 % w/v kefir grains at the temperature of 25 °C possessed a higher reducing power than others expressed as absorbance at 700 nm (0.951 ± 0.032) and followed by fermented beverage with 8 % w/v kefir grains at temperature of 19 °C and 5 % w/v kefir grains at temperature of 25 °C. The lowest reducing power in fermented beverage was 0.660 ± 0.038 (absorbance at 700 nm) observed in sample prepared with 5 % w/v kefir grains at 19 °C. Changes in reducing power of fermented beverages are depicted in Fig. 4. It can be seen reducing power enhanced as the fermentation temperature increased. Furthermore, data in Table 1 show fermentation with kefir grains was also noted to increase significantly (p < 0.05) the reducing activity of beverage expressed as compared to unfermented beverage. Like DPPH radical scavenging activity type of fermentation could affect the reducing power (Table 1) and similar results were obtained about the effect of fermentation temperature and kefir grains inoculation on reducing power.
Fig. 4.
Changes in reducing power of beverages during the fermentation process. Fermentation temperature of 19 °C: open symbol, Fermentation temperature of 25 °C: closed symbol, kefir inoculation of 5 % w/v: circle, kefir inoculation of 8 % w/v: square. Bars represent the standard deviation
It has been reported by Liu et al. (2005) the reducing power of both milk and soymilk increased significantly after fermentation by kefir grains. Wong and Kitts (2003) attributed reducing activity of certain butter milk solids to the sulfhydryl and free hydroxyl groups. Whey is a rich source of sulfhydryl amino acids such as cysteine and liberation of cysteine during fermentation can increase reducing power. In addition, formation of reductants during fermentation may be another reason for the increase in reducing ability. These reductants can react with free radicals, stabilize them and suppress radical chain reactions. It has been reported reducing power of some lactic acid bacteria is excellent (Lin and Yen 1999). Increase in reducing power may be also contributed to the intracellular antioxidants, peptides of the starter organisms and their hydrogen-donating ability (Yang et al. 2000). Besides, Pomegranate is a rich source of phenolic components and Khanizadeh et al. (2008) showed correlation between phenolic components and antioxidant activity. After fermentation TPC increased too, this could lead to increase in the reducing power.
Inhibition effect upon linoleic acid autoxidation
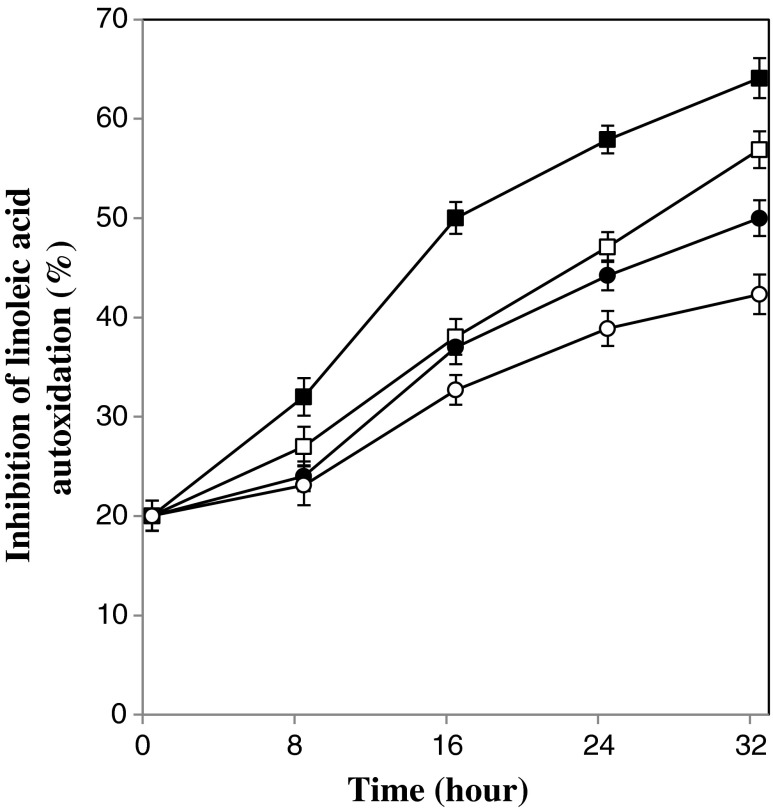
Results of inhibition effect upon linoleic acid autoxidation are shown in Table 1. It can be observed all fermented beverages significantly (p < 0.05) showed higher inhibition rate as compared with the control sample and the highest rate was observed for the beverage fermented by 8 %w/v kefir grains at temperature of 25 °C (64.09 ± 2 %). Also, Fig. 5 shows the changes of inhibition effect upon linoleic acid autoxidation during fermentation. The highest inhibition rate was obtained after 32 h of fermentation. It means inhibition effect upon linoleic acid autoxidation for all beverages increased during fermentation process.
Fig. 5.
Changes in inhibition effect upon linoleic acid autoxidation during the fermentation process. Fermentation temperature of 19 °C: open symbol, Fermentation temperature of 25 °C: closed symbol, kefir inoculation of 5 % w/v: circle, kefir inoculation of 8 % w/v: square. Bars represent the standard deviation
It has been previously reported that fermentation by kefir grains could increase inhibitory effect of milk and soy milk upon linoleic acid peroxidation (Liu et al. 2005). Pena-Ramos and Xiong (2001) showed that peptides deriving from milk protein hydrolysates inhibited oxidation and they related this effect to the specific amino acid residue side-chain groups or the specific peptide structure of the antioxidative peptides. Thus, we suggest that the peptides deriving from whey proteins might be contributed to the increase of inhibitory effects of beverage upon lipid peroxidation.
Inhibitory effect upon ascorbate autoxidation
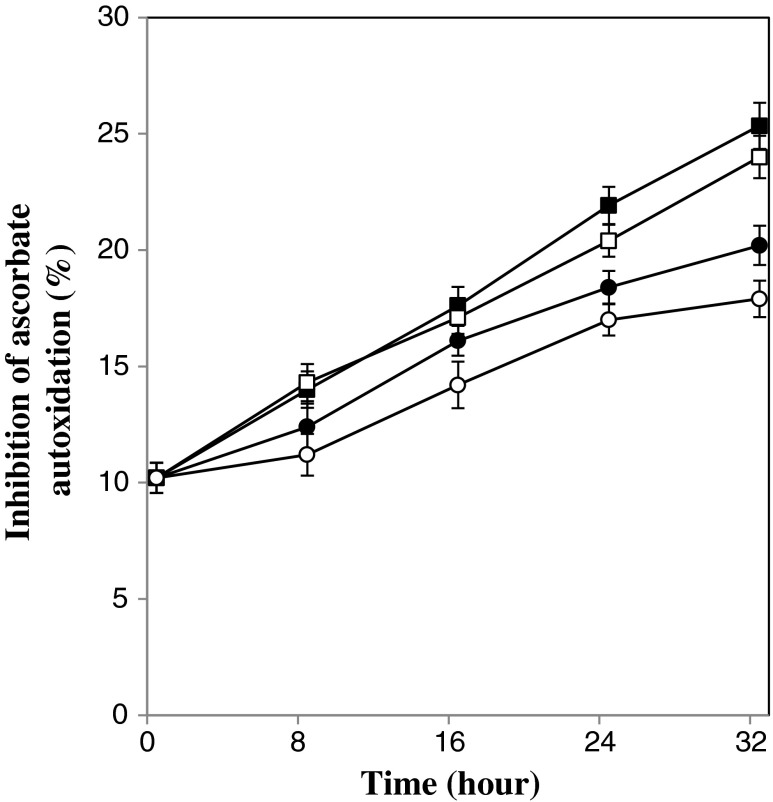
Table 1 shows that fermentation of beverage with kefir grains significantly (p < 0.05) increased the inhibition rate of ascorbate autoxidation. Also, Fig. 6 shows the change of inhibition effect upon ascorbate autoxidation during 32 h of fermentation. It can be observed in all beverages the inhibition effect upon ascorbate autoxidation increased as the temperature enhanced but this change depended on type of fermentation. At higher level of kefir grain amount, different temperature couldn’t have significant (p < 0.05) effect upon inhibitory effect, but when lower level of kefir grains (5 %w/v) was applied it was observed at temperature of 25 °C inhibitory effect was higher as compared with 19 °C. The inhibition of ascorbate autoxidation observed with beverage may be attributed to the phenols found in pomegranate juice. Wang et al. (2006) found soymilk exhibited inhibition of ascorbate autoxidation and they attributed this to soybean phenol components. Liberation of some phenol content (Chien 2004) throughout the some enzymatic catalytic actions and intracellular antioxidants of starter organisms (Lin and Yen 1999) may be accounted for the increase of ascorbate autoxidation inhibition.
Fig. 6.
Changes in inhibition effect upon ascorbate autoxidation during the fermentation process. Fermentation temperature of 19 °C: open symbol, Fermentation temperature of 25 °C: closed symbol, kefir inoculation of 5 % w/v: circle, kefir inoculation of 8 % w/v: square. Bars represent the standard deviation
Conclusion
The results of this survey confirmed kefir grains fermentation of present beverage showed good results of TPC and antioxidant activities. Also it can be said mixture of whey and pomegranate juice could be used as new substrate for producing novel fermented beverage with kefir grains. Generally, TPC, DPPH radical scavenging, reducing power, inhibition effect upon linoleic acid autoxidation and inhibition effect upon ascorbate autoxidation increased after fermentation but no significant change was observed about metal chelating activity. On the basis of this study it was obtained fermentation with kefir grains has beneficial effects on beverage since it can enhances the antioxidant activities. So, more research can be done for producing novel fermented beverage by kefir grains. Also, more uses of whey can be applied to change it from a waste to a delicious beverage.
Acknowledgments
The authors would like to acknowledge the assistance of microbiology and chemistry laboratory from the food science department of University of Tehran throughout the research project.
Footnotes
Highlight
• Fermentation by kefir grains had the ability to enhance TPC.
• Fermentation could enhance antioxidant activities except metal chelating effect.
•Temperature and inoculation level could have effect on TPC and antioxidant activity changes.
• Pomegranate juice and whey was a suitable media for producing a novel dairy-juice beverage.
Contributor Information
Nayereh Sabokbar, Phone: 0+989123554212, Email: nayereh.sabokbar@yahoo.com.
Faramarz Khodaiyan, Phone: 0+989123113195, Email: Khodaiyan@ut.ac.ir.
References
- Boskov-Hansen H, Andersen MF, Nielsen LM, Back-Knudsen KE, Meyer AS, Christensen LP, Hansen A. Changes in dietary fibre, phenolic acids and activity of endogenous enzymes during rye bread making. Eur Food Res Technol. 2002;214:33–42. doi: 10.1007/s00217-001-0417-6. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chatterton DEW, Smithers G, Roupas P, Brodkorb A. Bioactivity of β-lactoglobulin and α-lactalbumin-technological implications for processing. Int Dairy J. 2006;16:1229–1240. doi: 10.1016/j.idairyj.2006.06.001. [DOI] [Google Scholar]
- Chien HL (2004) Change of isoflavones contents in cultured soymilk fermented with lactic acid bacteria and bifidobacteria. M.S. Thesis, National Taiwan University, Taipei
- Coda R, Larena A, Trani A, Gobbetti M, Cagno RD. Yogurt-like beverages made of a mixture of cereals, soy and grape must: microbiology, texture, nutritional and sensory properties. Int J Food Microbiol. 2012;155:120–127. doi: 10.1016/j.ijfoodmicro.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 1990;38:674–677. doi: 10.1021/jf00093a019. [DOI] [Google Scholar]
- Dordević TM, Šiler-Marinković SS, Dimitrijević-Brankovic SI. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010;119:957–963. doi: 10.1016/j.foodchem.2009.07.049. [DOI] [Google Scholar]
- Fawole OA, Opara UL, Theron KI. Chemical and phytochemical properties and antioxidantactivities of three pomegranate cultivars grown in South Africa. Food Bioprocess Technol. 2011 [Google Scholar]
- Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals, and disease. Biochem J. 1984;219:1–4. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Ledesma B, Miralles B, Amigo L, Ramos M, Recio I. Identification of antioxidant and ACE-inhibitory peptides in fermented milk. J Sci Food Agric. 2005;85:1041–1048. doi: 10.1002/jsfa.2063. [DOI] [Google Scholar]
- Kehrer JP. Free radicals as mediators of tissue injury and disease. CRC Crit Rev Toxicol. 1993;23:21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- Khanizadeh S, Tsao R, Rekika D, Yang R, Charles MT, Rupasinghe HPV. Polyphenol composition and total antioxidant capacity of selected apple genotypes for processing. J Food Compos Anal. 2008;21:396–401. doi: 10.1016/j.jfca.2008.03.004. [DOI] [Google Scholar]
- La Riviére JWM, Kooiman P, Schmidt K. Kefiran, a novel polysaccharide produced in the kefir grain by lactobacillus brevis. Arch Microbiol. 1967;59:269–278. doi: 10.1007/BF00406340. [DOI] [PubMed] [Google Scholar]
- Lin MY, Chang FJ. Antioxidative effects of intestinal bacteria Bifidobacterium longum ATCC 15708 and Lactobacillus acidophilus ACTT 4356. Dig Dis Sci. 2000;45:1617–1622. doi: 10.1023/A:1005577330695. [DOI] [PubMed] [Google Scholar]
- Lin MY, Yen CL. Antioxidative ability of lactic acid bacteria. J Agric Food Chem. 1999;47:1460–1466. doi: 10.1021/jf981149l. [DOI] [PubMed] [Google Scholar]
- Liu JR, Chen MJ, Lin CW. Antimutagenic and antioxidant properties of milk kefir and soy-milk kefir. J Agric Food Chem. 2005;53:2467–2474. doi: 10.1021/jf048934k. [DOI] [PubMed] [Google Scholar]
- Lu Y, Foo LY. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000;68:81–85. doi: 10.1016/S0308-8146(99)00167-3. [DOI] [Google Scholar]
- Marazza JA, Nazareno MA, Giori GSD, Garro MS. Enhancement of the antioxidant capacity of soymilk by fermentation with Lactobacillus rhamnosus. J Funct Foods. 2012;4:594–601. doi: 10.1016/j.jff.2012.03.005. [DOI] [Google Scholar]
- Mishra OP, Kovachich GB. Inhibition of the autoxidation of ascorbate and norepinephrine by extracts of Clostridium butyricum, Megasphaera elsdenii and Escherichia coli. Life Sci. 1984;35:849–854. doi: 10.1016/0024-3205(84)90410-7. [DOI] [PubMed] [Google Scholar]
- Moran JF, Klucas RV, Grayer RJ, Abian J, Becana M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: prooxidant and antioxidant properties. Free Radic Biol Med. 1997;22:861–870. doi: 10.1016/S0891-5849(96)00426-1. [DOI] [PubMed] [Google Scholar]
- Mousavi ZE, Mousavi SM, Razavi SH, Emam-Djomeh Z, Kiani H. Fermentation of pomegranate juice by probiotic lactic acid bacteria. World J Microbiol Biotechnol. 2011;27:123–128. doi: 10.1007/s11274-010-0436-1. [DOI] [Google Scholar]
- Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Pena-Ramos EA, Xiong YL. Antioxidative activity of whey protein hydrolysates in a liposomal system. J Dairy Sci. 2001;84:2577–2583. doi: 10.3168/jds.S0022-0302(01)74711-X. [DOI] [PubMed] [Google Scholar]
- Permyakov AE, Berliner LJ. α-Lactalbumin: structure and function. FEBS Lett. 2000;473:269–274. doi: 10.1016/S0014-5793(00)01546-5. [DOI] [PubMed] [Google Scholar]
- Pokorny J, Schmidt S. The impact of food processing in phytochemicals: the case of antioxidants. Phytochem functi foods. Cambridge: Woodhead Publishing; 2003. p. 298. [Google Scholar]
- Sabokbar N, Khodaiyan F. Characterization of pomegranate juice and whey based novel beverage fermented by kefir grains. J Food Sci Technol. 2014 doi: 10.1007/s13197-014-1412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F, Wanasundara UN, Amarowicz R. Natural antioxidants from low-pungency mustard fiour. Food Res Int. 1994;27:489–493. doi: 10.1016/0963-9969(94)90244-5. [DOI] [Google Scholar]
- Simic MG. Mechanisms of inhibition of free-radical processed in mutagenesis and carcinogensis. Mutat Res. 1988;202:377–386. doi: 10.1016/0027-5107(88)90199-6. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JAJ. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Suetsuna K, Ukeda H, Ochi H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J Nutr Biochem. 2000;11:128–131. doi: 10.1016/S0955-2863(99)00083-2. [DOI] [PubMed] [Google Scholar]
- Sun YP, Chou CC, Yu RC. Antioxidant activity of lactic-fermented Chinese cabbage. Food Chem. 2009;115:912–917. doi: 10.1016/j.foodchem.2008.12.097. [DOI] [Google Scholar]
- Tong LM, Sasaki S, Julian McClements D, Decker EA. Mechanisms of the antioxidant activity of a high molecular weight fraction of whey. J Agric Food Chem. 2000;48:1473–1478. doi: 10.1021/jf991342v. [DOI] [PubMed] [Google Scholar]
- Voung T, Martin L, Matar C. Antioxidant activity of fermented berry juices and their effects on nitric oxide and tumor necrosis factor-alpha production in macrophages 264.7 gamma NO (-) cell line. J Food Biochem. 2006;30:249–268. doi: 10.1111/j.1745-4514.2006.00054.x. [DOI] [Google Scholar]
- Wang YC, Yu RC, Chou CC. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006;23:128–135. doi: 10.1016/j.fm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Wong PYY, Kitts DD. Chemistry of buttermilk solid antioxidant activity. J Dairy Sci. 2003;86:1541–1547. doi: 10.3168/jds.S0022-0302(03)73739-4. [DOI] [PubMed] [Google Scholar]
- Yang JH, Mau JL, Ko PT, Huang LC. Antioxidant properties of fermented soybean broth. Food Chem. 2000;71:249–254. doi: 10.1016/S0308-8146(00)00165-5. [DOI] [Google Scholar]
- Yen GC, Duh PD, Chuang DY. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000;70:437–441. doi: 10.1016/S0308-8146(00)00108-4. [DOI] [Google Scholar]
- Zaouay F, Mena P, Garcia-Viguera C, Mars M. Antioxidant activity and physico-chemical properties of Tunisian grown pomegranate (Punica granatum L.) cultivars. Ind Crop Prod. 2012;40:81–89. doi: 10.1016/j.indcrop.2012.02.045. [DOI] [Google Scholar]