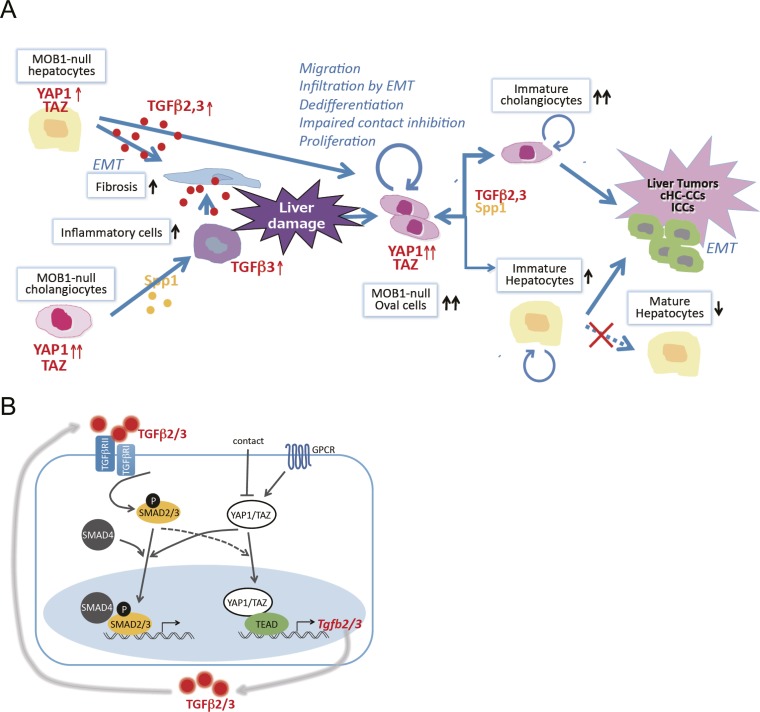
Fig. S7.
Molecular explanation. (A) Model of a molecular explanation for the phenotypes observed in Mob1a/1b-deficient liver. MOB1 deficiency increases the production of cytokines such as TGF-β2,3 and SPP1, which increase fibrosis and inflammatory cells in the liver. Liver damage by fibrosis and inflammatory cells ensues that induces the appearance of oval cells. Activation of YAP1 in oval cells in the absence of MOB1A/1B stimulates cell proliferation, impairs contact inhibition, promotes dedifferentiation resulting in stem/progenitor cell maintenance, and facilitates oval cell infiltration by EMT throughout the liver. TGF-β2,3 also positively regulate YAP1 activation, and the increased TGF-β2,3 and SPP1 present promote a lineage shift from hepatocytes to cholangiocyte-like cells. Under the continuing influence of highly activated YAP1/TAZ, these oval cells and immature cholangiocytes continue to proliferate and eventually undergo malignant transformation. Liver tumors, particularly cHC-CCs and ICCs, are then generated in LMob1DKO mice and soon show enhanced EMT invasion. (B) Cross-talk between the YAP1/TAZ and TGF-βs–SMADs signaling pathways. Engagement of the TGF-βR complex by TGF-β2/3 leads to SMAD2/3 activation, interaction with SMAD4, and translocation of the SMAD complex into the nucleus, where it activates transcription of TGF-β2/3 target genes. Engagement of GPCR inhibits the Hippo pathway leading to YAP1/TAZ activation, a process inhibited by contact inhibition. Nuclear translocation of activated YAP1/TAZ both increases TEAD-mediated transcription of TGF-βs and enhances nuclear translocation of SMAD2/3. Reciprocally, TGF-β2/3-mediated activation of SMAD2/3 may activate YAP1/TAZ and promote its nuclear translocation. Thus, positive cross-talk is observed between the TGF-βs–SMADs pathway and YAP1/TAZ signaling.