Figure 4.
Mechanism of G4 Bypass by PrimPol
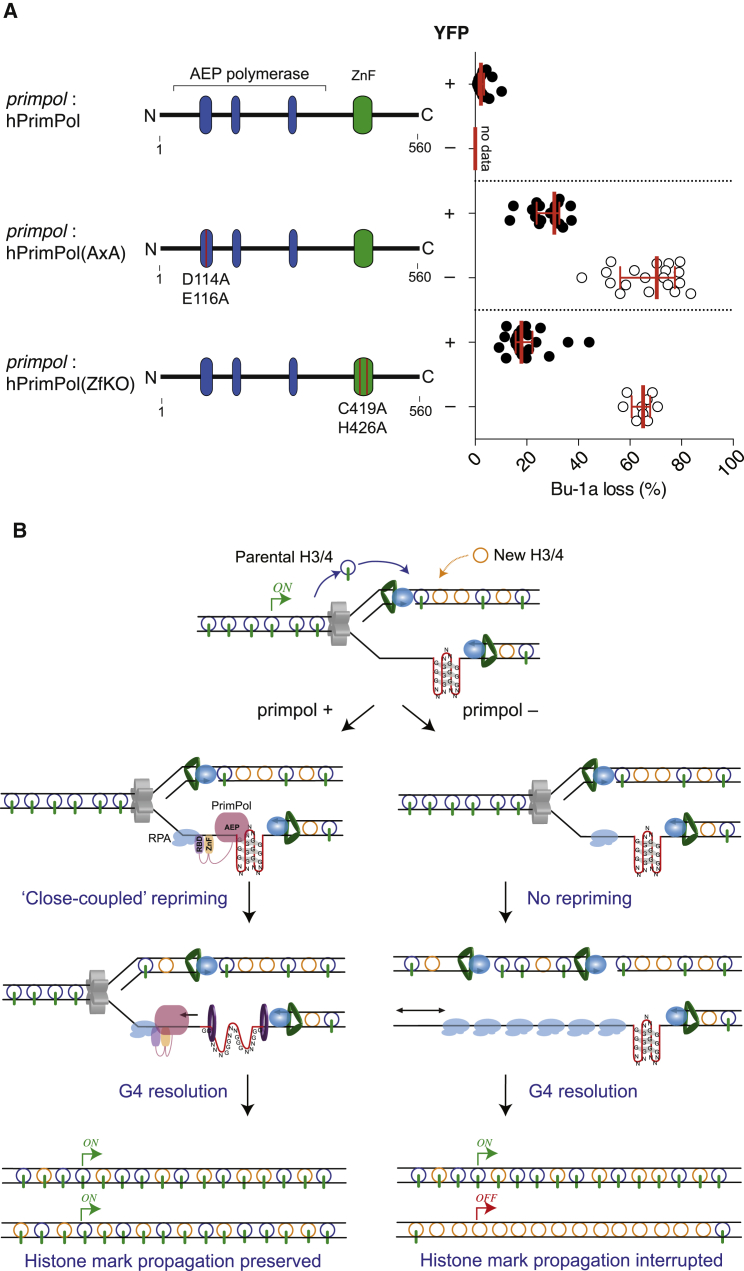
(A) Complementation of primpol cells with human PrimPol. PrimPol, or mutants, tagged with YFP were expressed in primpol cells and single YFP +ve/Bu-1a +ve clones were expanded for 3 weeks. In order to exclude cells that had lost expression of PrimPol during expansion, FACS analysis for Bu-1a expression was performed after gating for YFP expression. The fluctuation analyses are presented separately for YFP +ve (filled circles) and YFP –ve (open circles) cells. Wild-type PrimPol was expressed stably so YFP-ve cells were not generated.
(B) A G4 blocks the leading strand polymerase resulting in exposure initially of a short tract of single-stranded DNA ahead of the G4, to which RPA binds. If PrimPol is present, it can be recruited to the RPA and adjacent ssDNA through its C-terminal RBD and ZnF domains. Binding of the catalytic domain to the G4 may also help localize the enzyme. PrimPol synthesizes a short primer adjacent to the G4 and this allows replication to continue, maintaining coupling of DNA synthesis with the advancing helicase and with histone recycling and thus the parental epigenetic state of the locus is maintained as the cells divide. If PrimPol is absent, there is no repriming resulting in a much longer tract of ssDNA being exposed at the G4. This results in significant displacement of parental histones. The resulting gap may be replicated by a fork arriving from the other direction or by eventual release of the blocked polymerase as the structure is unwound. In either case, if the gap is replicated without the supply of parental histones, the pre-existing epigenetic state is lost. See also Figure S4.