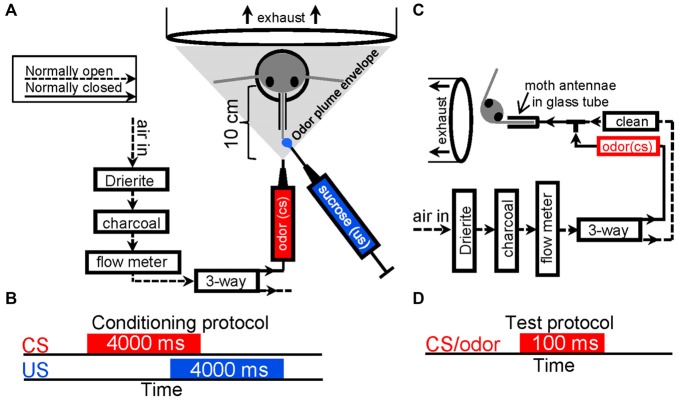
Figure 1.
Stimulus delivery protocols. (A) Schematic of the odor delivery system with relationship to the moths head. Arrows indicate the path of airflow through the olfactometer. House compressed air was first passed through 500 cc’s Drierite and a 500 cc’s active charcoal before passing through a flow meter. From the flow meter, clean air then passed into a three-way valve then out of the normally open output unused. During conditioning, odor (the conditioned stimulus or CS) was presented to the antenna by actuating the valve and shunting airflow to the normally closed output, into an odor cartridge (red), then out of a 1 mm ID nozzle at a velocity of 3.3 m/s. The nozzle was positioned approximately 10 cm in front of the moth head; ejected odor spread creating a conical envelop (gray triangle) that covered and was pulled over the antennae and into an exhaust located behind the head at a measured velocity of 0.3 m/s. Sucrose solution (blue; the unconditioned stimulus or US) was presented manually to the partially extended and restrained proboscis using a Gilmont type syringe. (B) A schematic depiction of the CS-US timing during conditioning. (C) A modified version of the stimulus delivery system described in (A) used during behavioral testing and intracellular recordings. Here the normally open output from the three-way valve was tied into a T-fitting that also received the output of the normally closed output line, which contained the odor cartridge. The third branch of the T was then attached to a glass sleeve that was placed over a single antenna. Thus, clean or odor laden airflow was constantly passing over the antenna. All expelled output from the odor delivery system was captured in an exhaust vent. (D) Schematic depiction of the test stimulus duration.