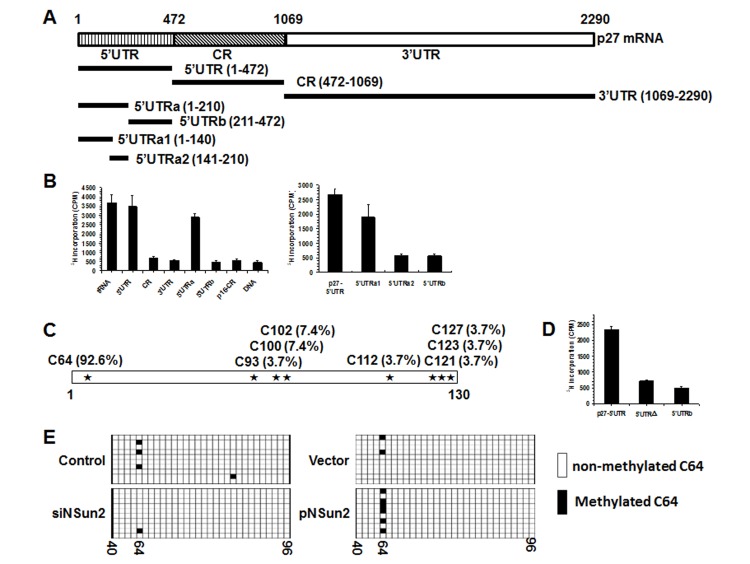
Figure 2. NSun2 methylates p27 5′UTR in vitro and in cells.
(A) Schematic representation depicting the p27 mRNA fragments used for in vitro methylation assays. (B) Incorporation of 3H-labeled SAM into p27 5′UTR, CR, 3′UTR, 5′UTRa, and 5′UTRb fragments (left) as well as 5′UTR, 5′UTRa1, 5′UTRa2, and 5′UTRb fragments (right). The incorporation of 3H-labeled SAM into p27 cDNA (DNA) and p16-CR (coding region) served as negative controls. The incorporation of 3H-labeled SAM into bacteria tRNA served as a positive control. (C) In vitro methylated 5′UTRa1 fragment was subjected to bisulfate RNA sequencing analysis to identify the methylation sites, as described in “Materials and Methods”. The percent of methylation at different sites is indicated. (D) Incorporation of 3H-labeled SAM into the 5′UTR variant mutating C64 (5′UTRΔ). 5′UTR and 5′UTRb served as negative and positive controls, respectively. (E) HeLa cells were transfected with a pGL3-derived reporter bearing the p27 5′UTR; 24 h later, cells were further transfected with a vector expressing NSun2 or with NSun2 siRNA and cultured for an additional 48 h. RNA was isolated and subjected to bisulfate sequencing analysis to assess the rate of C64 methylation. Open boxes indicate cytosine-to-uracil conversion, read as thymidine in the cDNA (unmethylated), and filled boxes indicate a retained cytosine (methylated). The numbers below the columns refer to cytosine positions in the p27 5′UTR.