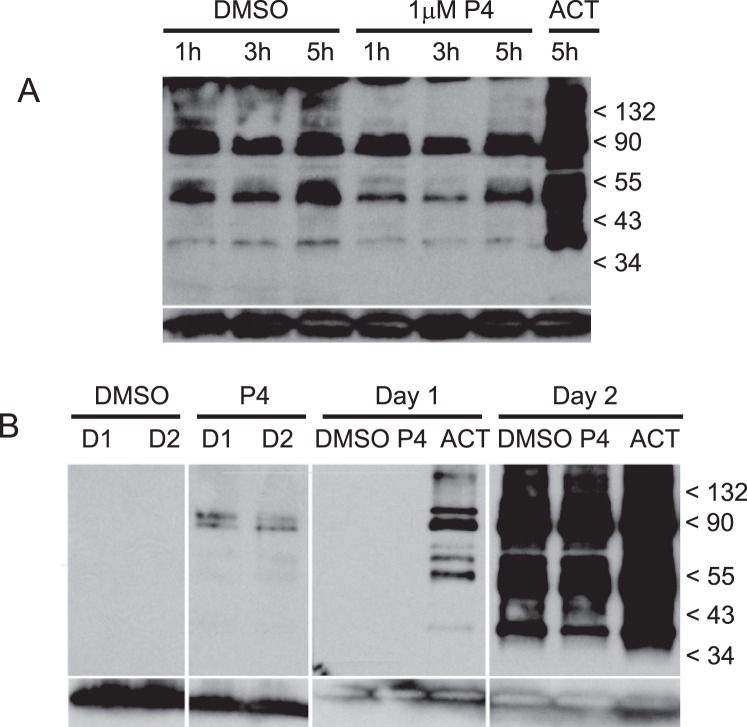
FIG. 4.
Tyrosine-specific phosphorylation of sperm proteins. Following incubation in capacitation medium, which follows the standard overnight incubation protocol (second day of incubation), sperm were treated with progesterone and then solubilized for Western blots (A). Whole sperm protein was separated by PAGE, blotted onto PVDF membranes, and probed with antiphosphotyrosine recombinant 4G10 mAb. Blot A contains the following sperm treatments; DMSO at 1, 3, and 5 h (lanes 1–3); 1 μM progesterone at 1, 3, and 5 h (lanes 4–6); and activator at 5 h (lane 7). Protein phosphorylation activity was compared between sperm incubated in capacitation medium on the same day as sample collection (first day) and following overnight incubation and subsequent transfer into capacitation medium (second day) (B). Blot B contains the following sperm treatments: DMSO at 0.25 h the first day and 0.25 h the second day (lanes 1 and 3, respectively); 1 μM progesterone at 0.25 h the first day and 0.25 h the second day (lanes 2 and 4, respectively); DMSO, 1 μM progesterone and activator at 5 h the first day (lanes 5–7, respectively); and DMSO, 1 μM progesterone and activator at 5 h the second day (lanes 8–10). Anti-human/mouse COX-4I1 antibody was used as a control for quantities of sperm proteins loaded on gels and blotted onto membranes (bottom row, A and B). A protein of approximately 20–22 kDa corresponding to COX-4I1 is shown at the bottom each lane.