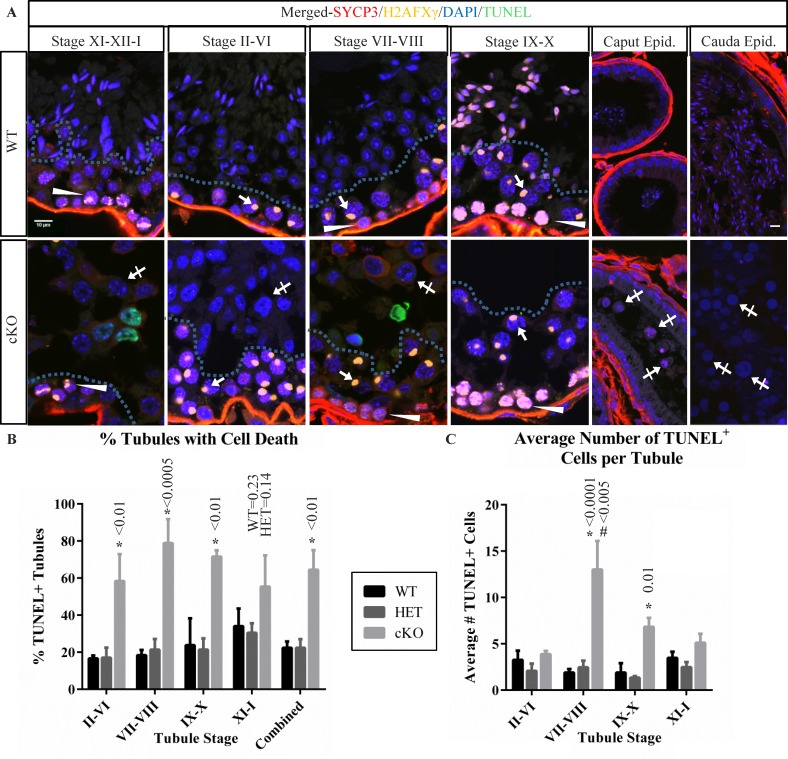
FIG. 6.
Germ cell populations in stages of cKO tubules indicate prolonged survival of meiotic arrested germ cells. A) Immunofluorescence localization of H2AFXγ (yellow) and SYCP3 (red), along with DAPI staining (blue) was used to group cKO seminiferous tubules into stage-groups and compared to WT. Arrows indicate H2AFXγ in pachytene-stage spermatocytes exhibiting X-Y body localization. Arrowheads indicate weak H2AFXγ chromatin localization in stage VII–VIII preleptotene-stage spermatocytes, strong labeling in stage IX–X leptotene-stage spermatocytes, and patchy labeling in stage XI–XII–I zygotene-stage and zygotene/pachytene-stage transitioning cells, characteristic of H2AFXγ relocalization to the forming XY body. Hashed lines in tubules of testis sections indicate the separation between spermatocytes in the basal portion of the seminiferous epithelium and the spermatids (WT) or abnormal arrested cells (cKO, crossed arrows) in the luminal portion. Cells contained in WT and cKO epididymis are also shown with abnormal cKO cells indicated (crossed arrows). Bars in the upper left and upper right panels are 10 μm and representative for all testis and epididymis panels. B) A bar graph summarizing the percent of seminiferous tubules containing TUNEL-positive cells in seminiferous tubules from stage groups II–VI, VII–VIII, IX–X, XI–I, and combined averages in testis sections from WT, HET, and cKO mice. C) A bar graph summarizing the number of TUNEL-positive cells per tubule containing such cells. Data for testis sections from each genotype and stage-group are indicated. Error bars represent SEM, asterisk (*) indicates statistically significant difference between genotypes at a given stage, # indicates statistically significant difference between stages for the given genotype. All indicated P-value calculations include Tukey multiple testing corrections.