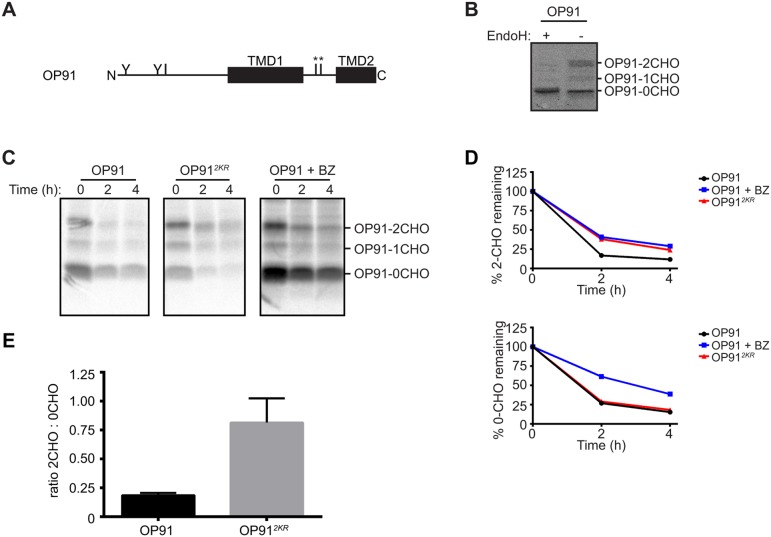
Fig. 7.
Cytoplasmic lysine residues are required for degradation of OP91, an ERAD substrate containing an unassembled TMD. (A) Schematic of the domain structure of OP91. Approximate location of N-glycosylation sites (Y) and lysine residues (I) are shown. * indicates cytoplasmic lysine residues replaced in OP912KR. (B) Lysates of HeLa cells transiently expressing OP91 were treated with EndoH or mock treated, then analysed by SDS-PAGE and immunoblotting with anti-opsin antibodies. (C) Cells expressing OP91 or OP912KR were pulse-labelled with [35S]Met/Cys for 60 min and chased for up to 4 h. OP91 was immunoprecipitated with anti-opsin antibodies and analysed by SDS-PAGE and phosphorimaging. Where indicated, cells were treated with bortezomib (BZ) throughout the pulse-chase labelling. (D) The amount of radiolabelled OP91 remaining at each time point was expressed relative to that present at the start of the chase. The glycosylated (2CHO) and non-glycosylated (0CHO) forms were quantified separately. Data represents the mean of two independent experiments. (E) The ratio of double glycosylated (2CHO) relative to non-glycosylated (0CHO) OP91 and OP912KR at steady state was quantified by immunoblotting with anti-opsin. The graph represents the mean±s.e.m. of four independent experiments. (C) The ratios of the double glycosylated ‘-2CHO’ to non-glycosylated ‘-0CHO’ forms of OP91 and OP912KR were quantified at t=0 (steady state). The graph represents the mean±s.e.m. of four independent experiments.