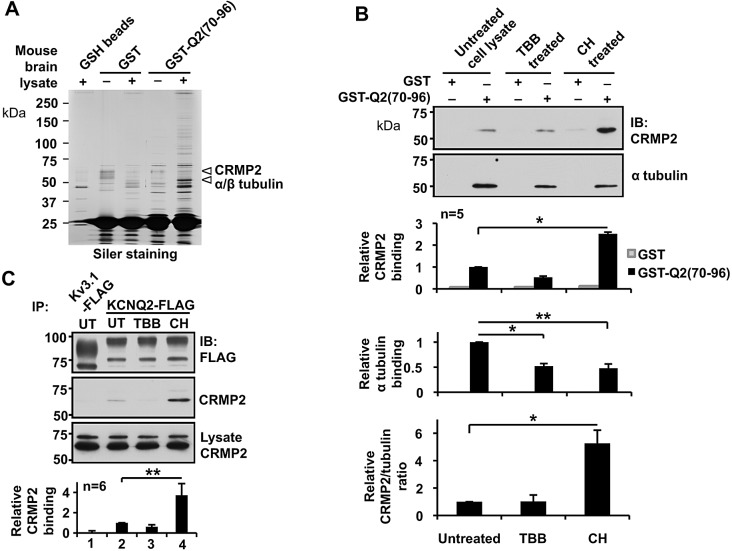
Fig. 5.
Identification and characterization of translocation-domain-binding proteins. (A) Representative silver-staining of an SDS-PAGE gel, showing selective purification by GST–Q2(70–96) fusion protein from mouse brain lysate. Pulldown assays using glutathione beads (GSH beads), GST-bound GSH beads (GST) and GST–Q2(70–96)-bound GSH beads [GST–Q2(70–96)] are shown. Arrowheads indicate GST–Q2(70–96)-purified bands used for mass spectrometric analyses. The highest ranked proteins from mass spectrometry analyses of each band are indicated. (B) Pulldown assays showing selective binding of GST–Q2(70–96) to CRMP-2 and α tubulin from untreated SH-SY5Y cell lysates; the binding was modulated by pre-treatment of cells with 5 µM TBB or 5 µM CHIR99021 (CH). *P<0.05, **P<0.01. IB, immunoblot; UT, untreated. (C) Immunoprecipitation of transiently expressed full-length KCNQ2–FLAG in SH-SY5Y cells, showing selective CRMP-2 binding and its modulation through pre-treatment with 5 µM TBB or 5 µM CHIR99021. **P<0.01. Error bars show s.e.m.