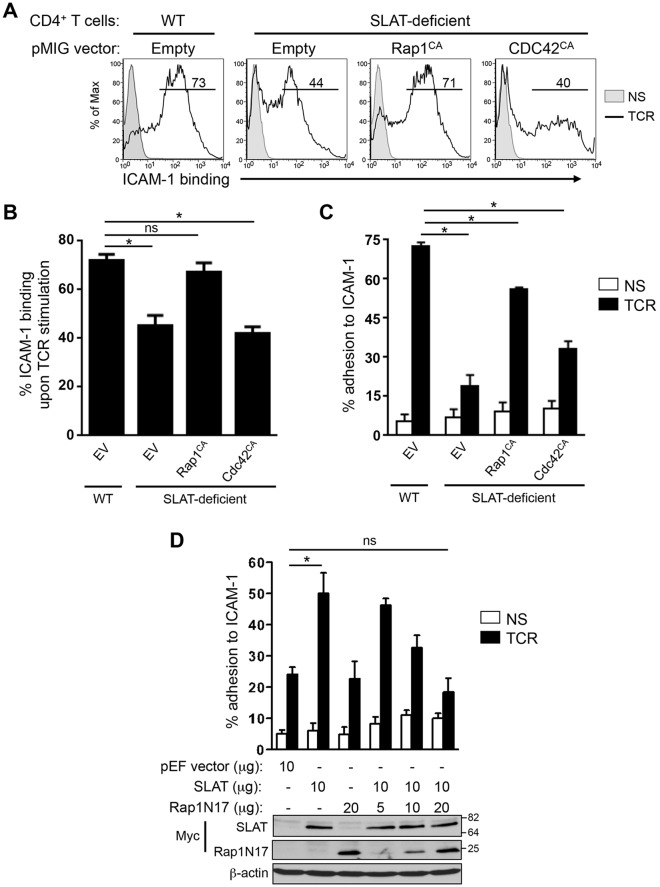
Fig. 5.
SLAT regulates TCR-mediated LFA-1 activation and T cell adhesion in a Rap1-dependent manner. (A–C) Primary WT and Def6−/− (KO) CD4+ T cells were activated with anti-CD3 and anti-CD28 mAbs plus IL-2, and transduced with retroviral pMIG vectors expressing either GFP alone (Empty) or GFP plus Rap1V12 (Rap1CA), or Cdc42Q61L (CDC42CA). Sorted GFP+ CD4+ T cells were left unstimulated (NS) or restimulated with anti-CD3 mAb (TCR) for 3 (A,B) or 45 (C) min, and analyzed for their ability to bind soluble Fc-ICAM-1 by flow cytometry (A,B) or adhere to plate-bound Fc-ICAM-1 (C). Numbers shown in FACS histograms (A) indicate the percentage of GFP+ CD4+ T cells that bound soluble Fc-ICAM-1 in a representative experiment, and the bar graphs shown in B and C represent the mean±s.d. of ICAM-1 binding of three independent experiments. (D) Jurkat JA16T cells were transfected with empty pEF vector or Myc–SLAT plasmids (10 µg each) along with dominant-negative Rap1 (Rap1N17) plasmid (5-20 µg). Cells were then either left unstimulated or stimulated for 45 min and subsequently analyzed for their ability to bind plate-bound Fc-ICAM-1. Lower panel, whole-cell lysates were immunoblotted with anti-Myc (SLAT and Rap1N17) and anti-β-actin antibodies to assess the expression of the transfected proteins and to control loading, respectively. Data are representative of three (A–C) and four (D) independent experiments. *P<0.01, WT versus Def6−/− cells; ns, not significant (two-tailed Student's t-test).