Abstract
We report on a patient with Rothmund–Thomson syndrome (RTS) whose cytogenetic evaluation showed a normal karyotype with no evidence of trisomy mosaicism or chromosomal rearrangements. Cultured lymphocytes from the patient, her mother, and a control exposed to mitomycin C and diepoxybutane did not show increased sensitivity to the dialkylating agents. Unlike some previous reports, we found no evidence of a deficiency in nucleotide excision repair, as measured with the functional unscheduled DNA synthesis assay. Glycophorin A analysis of red blood cells for somatic mutation revealed suspiciously high frequencies of both allele loss and loss-and-duplication variants in the blood of the patient, a pattern consistent with observations in other RecQ-related human diseases, and evidence for clonal expansion of a mutant clone in the mother. Discrepant results in the literature may reflect true heterogeneity in the disease or the fact that a consistent set of tests has not been applied to RTS patients.
Keywords: induced chromosome breakage, sister chromatid exchange, somatic mutation, unscheduled DNA synthesis
Rothmund–Thomson syndrome (RTS) is a rare autosomal recessive disorder with poikiloderma of the face and extremities beginning in infancy. Variable features include skeletal abnormalities, hypogonadism, juvenile cataracts, alopecia, retarded physical development, and a high incidence of cutaneous and noncutaneous malignancies (1). It has been postulated that a primary defect in DNA repair is responsible for the premature aging and cancer susceptibility associated with this syndrome, although no definitive test results have been reported. Conflicting results for chromosomal instability and sensitivity to ultraviolet (UV)-B irradiation have been reported (1). Mutations in a newly identified RecQ helicase gene at 8q24.3 have recently been identified in 3 of 7 RTS families (2, 3). We report on a patient with RTS who was analyzed using a variety of methodologies to investigate evidence of genomic instability.
Materials and methods
Case report
The patient is the only child born to her 23-year-old nonconsanguineous Puerto Rican parents. She has a healthy maternal half-brother. At delivery, she was small for 42 weeks gestation, with a birth weight of 2.03 kg and a length of 44.5 cm. Bilateral absence of the radii and thumbs and an umbilical hernia were noted at birth. Chromosome analysis was 46,XX. Medical complications during infancy included severe gastroesophageal reflux, recurrent infections, chronic microcytic hypochromic anemia requiring transfusions on three different occasions, and multiple orthopedic surgeries on her extremities. At 4 months of age, she developed pigmentary changes in her skin. At 1 year of age, the patient began to develop a reticular pattern of hyperpigmentation over her elbows and legs. The poikilodermal changes were consistent with RTS. Ophthalmologic examination and renal and abdominal ultrasounds were normal.
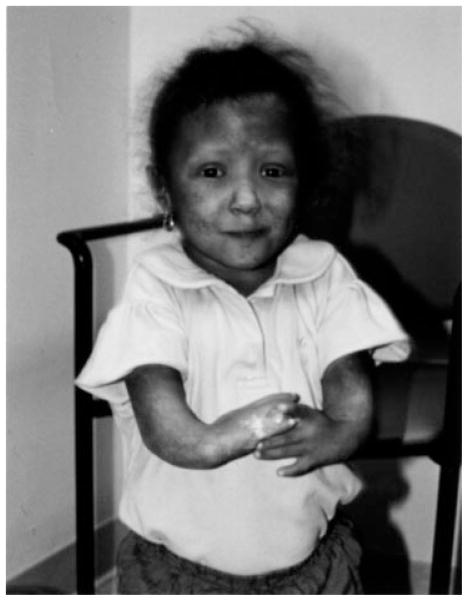
On physical examination at 4 years and 9 months (Fig. 1), her height was 82 cm (50th percentile for 18 months) and her weight was 11.6 kg, both well below two standard deviations of the mean for her age. Her head circumference was 49 cm, at the 25th percentile for her age. She had a flat nasal bridge, normal fundi, radial ray defects, and partial 2–3 syndactyly of the toes. Her skin showed poikilodermal changes over her entire body, including the face. The patient’s motor and speech development was delayed, but her social development was normal.
Fig. 1.
The patient at 5.25 years of age.
Cytogenetic evaluation
Human subject research was conducted under IRB protocol. Peripheral blood from the patient, her mother, and a normal control were cultured in Roswell Park Memorial Institute culture medium (RPMI) 1640 supplemented with 15% fetal bovine serum (FBS), 1% antibiotic, and 2% phytohemagglutinin for 72 h. Culture conditions included no treatment with and without 7.5 μg bromodeoxyuridine (BrdU) for 72 h, 50 ng/ml mitomycin C (MMC) for 72 h with and without BrdU (4, 5), and 0.1 μg/ml diepoxybutane (DEB) (6) for the last 48 h of culture. One hundred GTG-banded cells were analyzed for chromosome abnormalities from the untreated culture. One hundred Giemsa solid-stained metaphases were scored for chromosome breakage for each culture. Cultures treated with BrdU were stained for sister chromatid exchange (SCE) using fluorescence plus Giemsa methodology (5).
Unscheduled DNA synthesis (UDS)
Nucleotide excision repair (NER) capacity was measured using autoradiographic analysis of UDS (7). The protocol has been described previously (8) with modifications. Lymphocytes were extracted from whole blood samples by Ficoll–Paque gradient (Pharmacia, Piscataway, NJ, USA) and placed into culture on Matrigel (Becton Dickinson, Bedford, MA, USA) in RPMI medium containing 15% FBS and antibiotics. Primary cultures of human foreskin fibroblasts (FF) were run as standards for comparison in every experiment. After 3 days in culture without passaging, cells were irradiated for 12 s with 254 nm UV light at a fluence of 1.2 J/m2 per s, for a total dose of 14 J/m2. Mean grains per nucleus for each experimental sample were divided by the mean grain counts for the FF as a percentage of FF activity, the accepted standard.
Glycophorin A (GPA) assay
Both patient and maternal blood samples were confirmed as heterozygous for the M/N blood type. Samples were processed as previously described (9). The frequency per million of each class of variant (Vf) was calculated as the total number of events falling within each defined region of the histogram (N/Ø and N/N, respectively) divided by the total number of cells analyzed.
Results
Among 100 banded metaphase cells from the patient, four chromatid or chromosome breaks, one trisomy 8 cell, one trisomy 11 cell, and one endoreduplicated cell were identified. Chromosome breakage and SCE were similar for patient, mother, and control for untreated and/or DEB-treated cultures, and they were similarly elevated in MMC-exposed cultures (Table 1).
Table 1.
Cytogenetic findings
| % Breaks
|
Average SCE
|
||||
|---|---|---|---|---|---|
| Untreated | MMC | DEB | Untreated | MMC | |
| Control | 8 | 26 | 6 | 9.0 (6–14) | 50.5 (22–71) |
| Patient | 4 | 18 | 7 | 10.1 (6–15) | 44.2 (16–73) |
| Mother | 7 | 21 | 3 | 8.1 (3–14) | 53.0 (24–80) |
Percentage of breakage indicates chromatid or chromosome breaks among 100 cells. SCE was scored among 25 cells and averaged (ranges in parentheses).
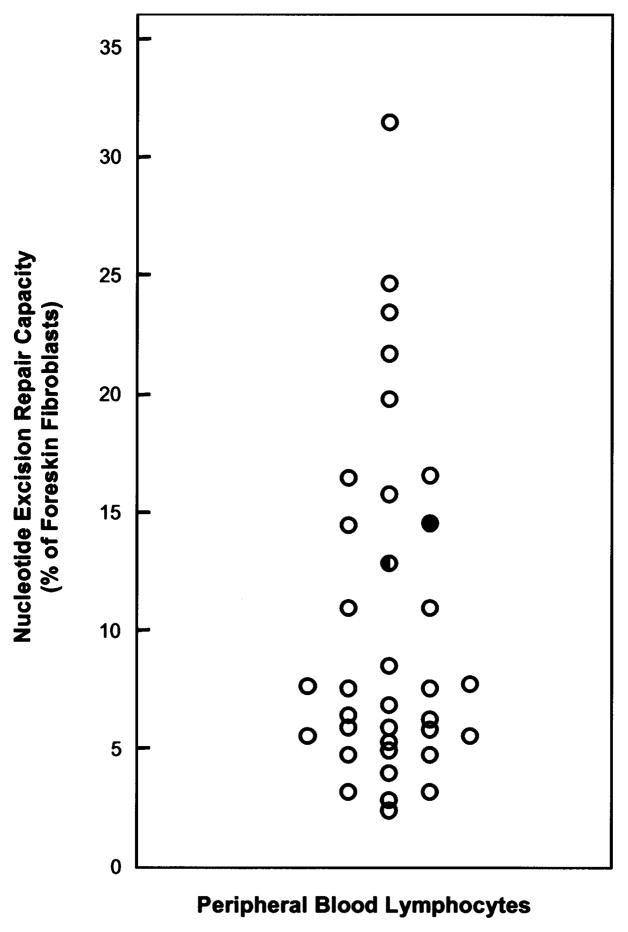
The patient’s and mother’s lymphocytes exhibited 14.0 and 12.3% of average normal FF NER capacity, respectively. Because levels of NER vary with tissues (8), a comparison was made with the level of NER measured in lymphocytes from 33 normal individuals (9.4±7.4% FF; Fig. 2). Thus, the patient actually exhibited 150% of average normal lymphocyte NER activity, and her mother exhibited 130%, indicating no NER deficiency. The slightly lower NER capacity of the 27-year-old mother’s lymphocytes is consistent with the observation that NER capacity decreases with age in normal individuals (10).
Fig. 2.
Comparison of the UDS capacities of the patient (filled symbol) and her mother (half-filled symbol) with a population of 33 normal individuals (age 32±11 years) with a range of values of 0.8–30.1% (mean 8.9±6.9%) (open symbols).
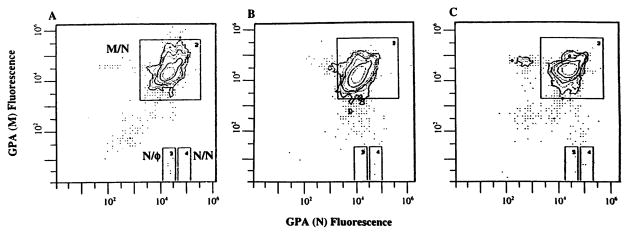
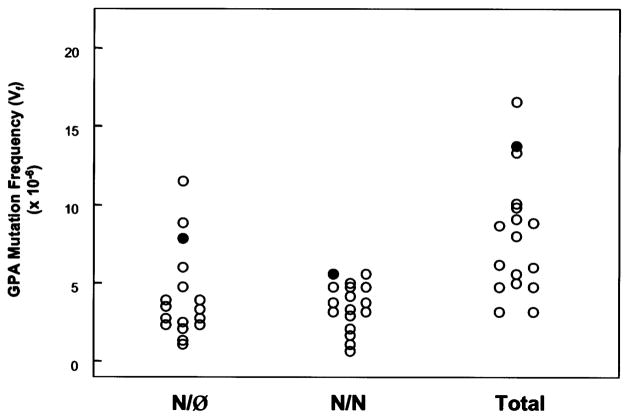
The frequency of GPA variant cells (Vf) for the patient and her mother’s sample were similar to previous estimates of ‘normal’ Vf for the GPA assay (Fig. 3; Table 2). Several studies with the GPA assay have demonstrated an age-dependence for somatic mutation (13, 14), with frequencies of both types of variant cells significantly increasing with age. It is therefore significant that the patient’s GPA Vf are both higher than her mother, who is older, and the best control in terms of sharing such factors as genetic background and environmental exposures with the patient. By comparison to pediatric controls (Table 2; Fig. 4), our patient’s results are in the high normal range for both types of mutations, as well as for total mutation. In terms of cumulative rank order for the two types of mutants, the patient is the highest of all the pediatric samples tested. The flow histogram of the GPA analysis of the mother’s blood sample reveals a distinct N-allele loss peak to the left of the main M/N peak containing an average of 1.46×10−4 variant cells, well above our threshold for an ‘outlier’ (Fig. 3), which occurs in ~2–5% of normal young adult individuals (11, 12, 15).
Fig. 3.
Flow distributions of labeling intensities from the analysis of 1×106 erythrocytes via the GPA assay from the concurrent normal control (A), the patient (B), and her mother (C). The vast majority of cells have the normal M/N phenotype and exhibit equal labeling with the M-specific and N-specific monoclonal antibodies. Directly below this main peak (at less than 1% labeling intensity) lies the N/Ø (allele loss) window, and to the immediate right, the N/N (loss-and-duplication) window (note log scales). The mother’s flow profile also exhibits a distinct peak of cells to the left of the normal peak (at ~1/3 intensity) corresponding to cells that have lost expression of the N-allele.
Table 2.
Frequency of somatic mutation at GPA N-allele
| Individual or population | n | Mean (±SD) | N/Ø Vf (×10−6) Median | Range | n | Mean±SD | N/N Vf (×10−6) median | Range |
|---|---|---|---|---|---|---|---|---|
| RTS patient | — | 8.0 | — | — | — | 5.8 | — | — |
| RTS mother | — | 4.6 | — | — | — | 3.2 | — | — |
| Local controls | 72 | 6.9±8.4 | 4.8 | 0.2–68.4 | 72 | 5.5±6.0 | 4.0 | 0.2–35.2 |
| 71a | 6.0±4.0 | 4.8 | 0.2–23.4 | |||||
| Pediatric controls | 16 | 4.2±2.7 | 3.3 | 1.4–11.6 | 16 | 3.7±1.4 | 3.8 | 1.0–5.8 |
| Jensen et al., 1991 (11) | 99 | 9.4±12.1 | 6.4 | 0.5–101.3 | 99 | 14.9±22.6 | 7.0 | 0.1–168.7 |
| 97b | 8.0±6.3 | 6.2 | 0.5–33.5 | 92c | 10.1±8.7 | 6.4 | 0.1–37.2 | |
| Manchester et al., 1995 (12) | 106 | 9.6±15.7 | 7.2 | 0.8–150.8 | 106 | 8.0±12.9 | 5.4 | 0.4–124.8 |
| 104d | 7.7±4.2 | 7.0 | 0.8–25.4 | 105e | 6.9±5.9 | 5.4 | 0.4–34.2 |
Excluding outlier with Vf of 68.4×10−6.
Excluding outliers with Vf of 52.0 and 101.3×10−6.
Excluding outliers with Vf of 41.8, 44.8, 47.4, 57.2, 60.2, 119.0, and 168.7×10−6.
Excluding outliers with Vf of 70.9 and 150.8×10−6.
Excluding outlier with Vf of 124.8×10−6.
Fig. 4.
Comparison of the GPA Vf of the patient (filled symbol) with a population of 16 pediatric controls (age 11±3 years) (open symbol). From left to right, N/Ø, N/N, and total Vf (N/Ø + N/N). Our patient shares the highest N/N Vf and has the third highest N/Ø Vf for a cumulative rank of 4; the next highest individuals, the child with the highest N/Ø and the other child with the highest N/N Vf, had cumulative rankings of 5 and 7, respectively.
Discussion
Our finding of one trisomy 8 cell may be relevant since trisomy 8 mosaicism has been a reported finding for some patients in cultured lymphocytes and/or fibroblasts (16–19). Evidence for in vivo trisomy 8 mosaicism was identified in two sibs in buccal mucosa and unstimulated lymphocytes (18). Other spontaneous abnormalities seen in patients have included isochromosomes involving 2q, 7q, 8q, and 21q, as well as balanced and unbalanced translocations (16–18, 20). Our patient did not show evidence of increased spontaneous or induced chromosomal breakage with exposure to MMC or DEB, and there was no increase in SCE, either spontaneously or after treatment with MMC.
Prior published results for chromosome instability and UDS have shown variable responses (Table 3), suggesting heterogeneity for RTS. The recent discovery of mutations in the RecQL4 gene in cell lines from some RTS patients implicates this helicase gene in the etiology of the disease (2, 3). Contradictory pieces of evidence suggest that RTS may be a heterogeneous disease, and that some cases of RTS are due to NER deficiency and that others are due to mutations in RecQL4 (or other RecQ-related genes). Another possibility is that RecQL4 is a previously undescribed component of the NER system itself; several of the known genes in the pathway code for DNA helicases, and there is a precedent for mutations in single NER genes to either affect DNA repair, producing a xeroderma pigmentosum (XP) phenotype, or to affect transcription, resulting in the Cockayne syndrome phenotype (31).
Table 3.
Assays of genomic instability and DNA repair capacity in RTS*
| Induced DNA repair | Chromosome instability | SCE | Somatic mutation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Ultraviolet light | Ionizing radiation | Spontaneous | MMC | Diepoxybutane | Bleomycin | Ionizing radiation | Spontaneous | MMC | Spontaneous | |
| Literature | Decreased [4] (21–23) | Increased [2] (26) | Increased [1] (19) (fibroblasts) | Increased [1] (27) | Normal [2] (18) | Increased [2] (28) | Increased [1] (29) | Normal [1] (30) (HPRT) | ||
| Normal [2] (24, 25) | Normal [2] (26) | Normal [4] (18, 19) (lymphocytes) | Normal [2] (18) | Normal [1] (18) | ||||||
| Our patient | Normal | Normal | Normal | Normal | Normal | Normal | Normal [?] (GPA) | |||
Numbers of patients are in square brackets and reference numbers are in round brackets.
Our results with the UDS assay suggest that the patient has normal NER capacity. Historically, most reports that evaluate NER in single patients are compared to a single concurrently analyzed normal control. These data are then reported as absolute grain counts or scintillation counts, which will vary with a number of factors, including the fluence of exposure, the radioactivity of the label, the time of development, the sensitivity of the emulsion, etc. Therefore, data from one experiment cannot be directly compared with that of another, precluding the accumulation of a database of comparable assay data.
Although previous studies have utilized lymphocytes, the vast majority of diagnostic applications of the UDS assay have been performed on cultured skin fibroblasts (32), since the obvious effects of UV exposure in XP patients are in the skin. Some studies, including that by Vasseur et al. (23), have observed similar effects in both skin fibroblasts and peripheral blood lymphocytes. Not only does the skin have a higher innate capacity for NER (making it easier to detect deficiencies), but it is far more likely to be exposed to UV radiation than bone marrow or circulating lymphocytes (33). Thus, for the fairly subtle deficiencies that have been reported for this disease (residual activity 20–70% of normal), it may well be that it is too difficult to document such decreases in lymphocytes to use them as a reliable basis for diagnostic assay.
A number of syndromes associated with premature aging and/or the increased incidence of cancer have been found to have underlying deficits in DNA repair, resulting in abnormally high frequencies of somatic mutation. The GPA assay has previously been used to demonstrate characteristically high frequencies of mutations in Bloom syndrome (BS) (34, 35), Fanconi anemia (FA) (36), ataxia telangiectasia (AT) (9, 37), and Werner syndrome (WS) (38, 39).
Although the GPA Vf observed for the patient and her mother are superficially normal, they are actually unusual enough to suggest an underlying defect in DNA repair. The patient exhibits high Vf relative to her mother and a population of pediatric controls (all older than our patient) for both the allele loss and loss-and-duplication classes of variants, although they are at the high range of the normal population. The other two known human diseases associated with mutations in human analogs of the RecQ helicase gene are BS and WS. They both show similarly increased levels of N/Ø and N/N, although very high (~100-fold) elevations in Vf were typically observed in patients with BS (34, 35) and relatively small (~ 2-fold) elevations were seen in the WS population (38). Thus, whereas the actual level of mutational increase may vary, the RecQ helicase-related diseases may be characterized by similar patterns of increased frequencies of both allele loss and loss-and-duplication variants.
The unusual GPA-labeling flow profile in the patient’s mother has been seen in sporadic unexplained outliers in normal populations (15) due to the clonal expansion of a hematopoietic stem cell carrying a single GPA mutation, as has been directly demonstrated for an HPRT-deficiency mutation in the lymphoid lineage (40). It is interesting to speculate that this clone may have undergone a loss of heterozygosity at the RTS locus, rendering it hypersensitive to further mutational events. This effect has been reported for BS and FA homozygotes where intragenic mitotic recombination has resulted in a population of revertant cells (41, 42).
We have presented a new case of RTS with normal levels of NER, suggesting that a deficiency of this type of DNA repair is not a causative factor in this particular patient, and that it may have subtly increased levels of somatic mutation, consistent with the pattern observed in other RecQ-related human diseases. With this continued evidence of possible heterogeneity within RTS, it is clear that a more standardized set of studies should be applied to individuals diagnosed with this disease, and that appropriate samples should be analyzed by experts in different aspects of the phenotype and correlated to the gene mutation in the gene or genes responsible for this disease.
Acknowledgments
The authors would like to thank the patient and her mother for their participation in this study. We also thank Lori Thomas, Michael Forlenza, Tariq Nazir, Elena Kisin, Karen Hanley-Yanez, Lisa Flowers, Linda Piersall, Lynn Janczukiewicz, and Kim Saksun for technical assistance with the UDS assay, and Manda Welsh and Britt Luccy for technical assistance with the GPA assay.
References
- 1.Vennos EM, Collins M, James WD. Rothmund–Thomson syndrome: review of the world literature. J Am Acad Dermatol. 1992;5:750–762. doi: 10.1016/0190-9622(92)70249-f. [DOI] [PubMed] [Google Scholar]
- 2.Kitao S, Shimamoto A, Goto M, et al. Mutations in RECQL4 cause a subset of cases of Rothmund–Thomson syndrome. Nature Genet. 1999;22:82–84. doi: 10.1038/8788. [DOI] [PubMed] [Google Scholar]
- 3.Lindor NM, Furuichi Y, Kitao S, Shimamoto A, Arndt C, Jalal S. Rothmund–Thomson syndrome due to RECQ4 helicase mutations: report and clinical and molecular comparisons with Bloom syndrome and Werner syndrome. Am J Med Genet. 2000;90:223–228. doi: 10.1002/(sici)1096-8628(20000131)90:3<223::aid-ajmg7>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Cervenka J, Arthur O, Yasis C. Mitomycin C test for diagnostic differentiation of idiopathic aplastic anemia and Fanconi anemia. Pediatrics. 1981;67:119–127. [PubMed] [Google Scholar]
- 5.Wenger SL. Chemical induction of sister chromatid exchange at fragile sites. Cancer Genet Cytogenet. 1995;85:72–74. doi: 10.1016/0165-4608(95)00137-9. [DOI] [PubMed] [Google Scholar]
- 6.Auerbach AD, Wolman SR. Susceptibility of Fanconi’s anaemia fibroblasts to chromosome breakage by DNA cross-linking agents. Nature. 1976;261:494–496. doi: 10.1038/261494a0. [DOI] [PubMed] [Google Scholar]
- 7.Cleaver JE, Thomas GH. The measurement of unscheduled DNA synthesis by autoradiography. In: Friedberg EC, Hanawalt PC, editors. Handbook of DNA repair techniques. New York: Marcel Dekker; 1981. pp. 269–287. [Google Scholar]
- 8.Latimer JJ, Hultner ML, Cleaver JE, Pedersen RA. Elevated DNA excision repair capacity in the extraembryonic mesoderm of the mid-gestation mouse embryo. Exp Cell Res. 1996;228:19–28. doi: 10.1006/excr.1996.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grant SG, Reeger W, Wenger SL. Diagnosis of ataxia telangiectasia with the glycophorin A somatic mutation assay. Genet Test. 1998;1:261–267. doi: 10.1089/gte.1997.1.261. [DOI] [PubMed] [Google Scholar]
- 10.Moriwaki S, Ray S, Tarone RE, Kraemer KH, Grossman L. The effect of donor age on the processing of UV-damaged DNA by cultured human cells: reduced DNA repair capacity and increased DNA mutability. Mutat Res. 1996;364:117–123. doi: 10.1016/0921-8777(96)00029-8. [DOI] [PubMed] [Google Scholar]
- 11.Jensen RH, Grant SG, Langlois RG, Bigbee WL. Somatic cell genotoxicity at the glycophorin A locus in humans. Prog Clin Biol Res. 1991;372:329–339. [PubMed] [Google Scholar]
- 12.Manchester DK, Nichlas JA, O’Neill JP, et al. Sensitivity of somatic mutations in human umbilical cord blood to maternal environments. Environ Mol Mutagen. 1995;26:203–212. doi: 10.1002/em.2850260304. [DOI] [PubMed] [Google Scholar]
- 13.Compton-Quintana PJE, Jensen RH, Bigbee WL, et al. Use of the glycophorin A human mutation assay to study workers exposed to styrene. Environ Health Perspect. 1993;99:297–301. doi: 10.1289/ehp.9399297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akiyama M, Kyoizumi S, Hirai Y, Kusonoki Y, Iwamoto KS, Nakamura N. Mutation frequency in human blood cells increases with age. Mutat Res. 1995;338:141–149. doi: 10.1016/0921-8734(95)00019-3. [DOI] [PubMed] [Google Scholar]
- 15.Bigbee WL, Fuscoe JC, Grant SG, et al. Human in vivo somatic mutation measured at two loci: individuals with stably elevated background erythrocyte glycophorin A (gpa) variant frequencies exhibit normal T-lymphocyte hprt mutant frequencies. Mutat Res. 1998;397:119–136. doi: 10.1016/s0027-5107(97)00186-3. [DOI] [PubMed] [Google Scholar]
- 16.Der Kaloustian VM, McGill JJ, Vekemans M, Kopelman HR. Clonal lines of aneuploid cells in Rothmund–Thomson syndrome. Am J Med Genet. 1990;37:336–339. doi: 10.1002/ajmg.1320370308. [DOI] [PubMed] [Google Scholar]
- 17.Ying KL, Oizumi J, Curry CJR. Rothmund–Thomson syndrome associated with trisomy 8 mosaicism. J Med Genet. 1990;27:258–260. doi: 10.1136/jmg.27.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindor NM, Devries EMG, Michels VV, et al. Rothmund–Thomson syndrome in siblings: evidence for acquired in vivo mosaicism. Clin Genet. 1996;49:124–129. doi: 10.1111/j.1399-0004.1996.tb03270.x. [DOI] [PubMed] [Google Scholar]
- 19.Miozzo M, Castorina P, Riva P, et al. Chromosomal instability in fibroblasts and mesenchymal tumors from 2 sibs with Rothmund–Thomson syndrome. Int J Cancer. 1998;77:504–510. doi: 10.1002/(sici)1097-0215(19980812)77:4<504::aid-ijc5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Orstavik KH, McFadden N, Hagelsteen J, Ormerod E, van der Hagen CB. Instability of lymphocyte chromosomes in a girl with Rothmund–Thomson syndrome. J Med Genet. 1994;31:570–572. doi: 10.1136/jmg.31.7.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinya A, Nishigori C, Moriwaki S-I, et al. A case of Rothmund–Thomson syndrome with reduced DNA repair capacity. Arch Dermatol. 1993;129:332–336. [PubMed] [Google Scholar]
- 22.Prache-de-Carrere B, Teillac-Hamel D, Capesius C, et al. Rothmund–Thomson syndrome with reduced DNA repair capacity. Ann Dermatol Venereol. 1996;123:395–397. [PubMed] [Google Scholar]
- 23.Vasseur F, Delaporte E, Zabot MT, et al. Excision repair defect in Rothmund Thomson syndrome. Acta Dermato-Venereologica. 1999;79:150–152. doi: 10.1080/000155599750011417. [DOI] [PubMed] [Google Scholar]
- 24.Cleaver JE. DNA damage and repair in light-sensitive human skin disease. J Invest Dermatol. 1970;54:181–195. doi: 10.1111/1523-1747.ep12280225. [DOI] [PubMed] [Google Scholar]
- 25.Jung EG. Investigations on dark repair in various light sensitive inherited disorders. Humangenetik. 1970;9:191–192. doi: 10.1007/BF00278935. [DOI] [PubMed] [Google Scholar]
- 26.Smith PJ, Paterson MC. Enhanced radiosensitivity and defective DNA repair in cultured fibroblasts derived from Rothmund Thomson syndrome patients. Mutat Res. 1982;94:213–228. doi: 10.1016/0027-5107(82)90183-x. [DOI] [PubMed] [Google Scholar]
- 27.Moss C, Bacon CJ, Mueller RF. “Isolated” radial ray defect may be due to Rothmund–Thomson syndrome. Clin Genet. 1990;38:318–319. [PubMed] [Google Scholar]
- 28.Kerr B, Ashcroft GS, Scott D, Horan MA, Ferguson MWJ, Donnai D. Rothmund–Thomson syndrome: two case reports show heterogeneous cutaneous abnormalities, an association with genetically programmed ageing changes, and increased chromosomal radiosensitivity. J Med Genet. 1996;33:928–934. doi: 10.1136/jmg.33.11.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melaragno MI, Smith MDAC. Sister chromatid exchange and proliferation pattern in lymphocytes from newborns, elderly subjects and in premature aging syndromes. Mech Ageing Devel. 1990;54:43–53. doi: 10.1016/0047-6374(90)90014-7. [DOI] [PubMed] [Google Scholar]
- 30.Lin YW, Kubota M, Akiyama Y, Sawada M, Furusho K. Measurement of mutation frequency at the HPRT locus in peripheral lymphocytes. Is this a good method to evaluate a cancer risk in pediatric patients? Adv Exp Med Biol. 1998;431:681–686. doi: 10.1007/978-1-4615-5381-6_132. [DOI] [PubMed] [Google Scholar]
- 31.Yu Z, Chen J, Ford BF, Brackley ME, Glickman BW. Human DNA repair systems: an overview. Environ Mol Mutagen. 1999;33:3–20. doi: 10.1002/(sici)1098-2280(1999)33:1<3::aid-em2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 32.Cleaver JE, Kraemer KH. Xeroderma pigmentosum. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. New York: Mc-Graw-Hill; 1989. pp. 2949–2971. [Google Scholar]
- 33.Otrin VR, McLenigan M, Takao M, Levine AS, Protic M. Translocation of a UV-damaged DNA binding protein into a tight association with chromatin after treatment of mammalian cells with UV light. J Cell Sci. 1997;110:1159–1168. doi: 10.1242/jcs.110.10.1159. [DOI] [PubMed] [Google Scholar]
- 34.Kyoizumi S, Nakamura N, Takebe H, Tatsumi K, German J, Akiyama M. Frequency of variant erythrocytes at the glycophorin-A locus in two Bloom’s syndrome patients. Mutat Res. 1989;214:215–222. doi: 10.1016/0027-5107(89)90166-8. [DOI] [PubMed] [Google Scholar]
- 35.Langlois RG, Bigbee WL, Jensen RH, German J. Evidence for elevated in vivo mutations and somatic recombination in Bloom’s syndrome. Proc Natl Acad Sci USA. 1989;86:670–674. doi: 10.1073/pnas.86.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sala-Trepat M, Boyse J, Richard P, Papadopoulo D, Moustacchi E. Frequencies of HPRT-lymphocytes and glycophorin A variants erythrocytes in Fanconi anemia patients, their parents and control donors. Mutat Res. 1993;289:115–126. doi: 10.1016/0027-5107(93)90137-5. [DOI] [PubMed] [Google Scholar]
- 37.Hewitt M, Mott MG. The assessment of in vivo somatic mutations in survivors of childhood malignancy. Br J Cancer. 1992;66:143–147. doi: 10.1038/bjc.1992.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyoizumi S, Kusunoki Y, Seyama T, Hatamochi A, Goto M. In vivo somatic mutations in Werner’s syndrome. Hum Genet. 1998;103:405–410. doi: 10.1007/s004390050841. [DOI] [PubMed] [Google Scholar]
- 39.Moser MJ, Bigbee WL, Grant SG, et al. Genetic instability and haematologic disease risk in Werner syndrome patients and heterozygotes. Cancer Res. 2000;60:2492–2496. [PubMed] [Google Scholar]
- 40.Nicklas JA, O’Neill JP, Sullivan LM, et al. Molecular analyses of in vivo hypoxanthine-guanine phosphoribosyl-transferase mutations in human T-lymphocytes: II. Demonstration of a clonal amplification of hprt mutant T-lymphocytes in vivo. Environ Mol Mutagen. 1988;12:271–284. doi: 10.1002/em.2860120302. [DOI] [PubMed] [Google Scholar]
- 41.Lo Ten Foe JR, Kwee ML, Rooimans MA, et al. Somatic mosaicism in Fanconi anemia: molecular basis and clinical significance. Eur J Hum Genet. 1997;5:137–148. [PubMed] [Google Scholar]
- 42.Ellis NA, Lennon DJ, Proytcheva M, Alhadeff B, Henderson EE, German J. Somatic intragenic recombination within the mutated locus BLM can correct the high sister-chromatid exchange phenotype of Bloom syndrome cells. Am J Hum Genet. 1995;57:1019–1027. [PMC free article] [PubMed] [Google Scholar]