Abstract
Hebeloma subsection Denudata includes the type of H. section Denudata, Hebeloma crustuliniforme, as well as the majority of the taxa commonly included in the Hebeloma crustuliniforme complex. Complementing the work of D.K. Aanen and co-workers, and using refined morphological and molecular methods we were able to recognize further individual taxa within the section. Fifteen species occurring in Europe are assigned to H. subsect. Denudata. Of these, we describe eight species as new, namely H. aanenii, H. aurantioumbrinum, H. geminatum, H. louiseae, H. luteicystidiatum, H. pallidolabiatum, H. perexiguum and H. salicicola. Naucoria bellotiana, a species very similar to H. alpinum is recombined into Hebeloma. A key to Hebeloma subsect. Denudata is provided. We demonstrate that within this subsection there is good overall consistency between morphological, phylogenetic and biological species concepts. In contrast to current opinion, in this group there is little species overlap, particularly when also considering species frequencies, between arctic and alpine floras on one hand and temperate on the other.
Keywords: Hebeloma eburneum, Hebeloma helodes, Hebeloma lutense, Hebeloma minus, Hebeloma pusillum, MCM7, mitochondrial SSU, Salix
INTRODUCTION
Hebeloma is a genus of ectomycorrhizal fungi occurring in many different habitats in the northern hemisphere (Marmeisse et al. 1999) and indeed worldwide, with the possible exception of regions, where Fagales are not endogenous, such as Northern South America (Tedersoo et al. 2010) or Africa. However, as typical nursery fungi, Hebeloma spp. are likely to have been introduced through human activity (see Sulzbacher et al. 2013).
Hebeloma crustuliniforme is one of the most often recorded (Vesterholt et al. 2014) species of the genus Hebeloma, but it is widely recognised that what has in the past been referred to as H. crustuliniforme is among the most notorious species complexes that have long defied recognition of individual taxa. In Vesterholt et al. (2014) we proposed an epitype for H. crustuliniforme so as to tie this name to a particular taxon within this complex, which we then suggested should be referred to as H. crustuliniforme (Bull.) Quél. emend. Vesterh., U. Eberh. & Beker to avoid confusion with earlier applied concepts of the taxon. As we stated at the time, this was the first step towards unravelling this complex of species within sect. Denudata.
More than 10 years ago, D.K. Aanen and others (Aanen & Kuyper 1999, 2004, Aanen et al. 2000) carried out a profound study of the H. crustuliniforme complex, using three different approaches, sporocarp morphology, molecular studies and intercompatibility tests, testing for dikaryotization between pairs of monokaryotic strains. They found 20 intercompatibility groups (ICGs) within the complex. A small number of strains were intercompatible with some strains of other ICGs or could not be unambiguously assigned to a unique ICG.
It is tempting to consider ICGs as biological species and they may well represent biological species, but as Aanen & Kuyper (1999) point out, the production of basidiospores in the dikaryotic mycelia generated in the intercompatibility tests could not be tested, therefore we do not know what the implications of the observed intercompatibility in nature are. In general, on the population level, partial intercompatibility may correspond to incomplete speciation (Aanen & Kuyper 1999). Almost all of a quite high number of strains stemming from 110 collections could be unambiguously assigned to a single ICG, thus supporting the notion that distinct biological taxa have already formed in this species aggregate.
Aanen & Kuyper (1999) linked this finding of partial compatibility between some ICGs with the difficulty of morphologically separating ICGs into morpho-species, suggesting that partial intercompatibility might contribute to the failure to form distinct morphological and ecological syndromes. Accordingly, Aanen and co-workers (Aanen 1999, Aanen et al. 2000, Aanen & Kuyper 2004) adopted wide species concepts, i.e. H. crustulinifome including H. alpinum with six ICGs and H. pusillum (four ICGs), partially overlapping with H. helodes (at least six ICGs). In the later work (Aanen & Kuyper 2004) H. helodes and H. pusillum were merged.
Molecular studies showed that three of the ICGs of the H. crustuliniforme complex were molecularly quite distinct from the other 17 ICGs. This supported a view published initially by Boekhout (1982), then molecularly supported (Aanen et al. 2000, Boyle et al. 2006), suggesting that there are indeed two distinct groups within this complex, one species complex around H. velutipes (syn. H. leucosarx acc. Grilli 2007) and another in sect. Denudata, around H. crustuliniforme. It has found its way into the classification system by Vesterholt (2005), which recognized two distinct sections Velutipes and Denudata, each including taxa that were formerly considered representatives of the H. crustuliniforme complex.
The focus of the present study is on H. sect. Denudata and particularly on members of the H. crustuliniforme complex and related taxa assigned to subsect. Denudata (described more fully below) of which H. crustuliniforme is the type. The delimitation of H. subsect. Denudata adopted in this paper is based on morphological characters. Within the scope of this paper we do not demonstrate the molecular delimitation of H. subsect. Denudata. A separate publication (Eberhardt et al. in prep.) will be concerned with the classification of subsections of H. sect. Denudata and species from these other subsections.
With regard to species delimitation, we follow de Queiroz (2007) unified species concept that treats different criteria such as morphological distinctness, monophyly, evolutionary distance or intercompatibility as independent lines of evidence for recognizing separately evolving lineages, i.e. species. This implies that not all lines of evidence necessarily support a species or the assignment of a particular specimen to a species, particularly in young species or species with a recent history of range changes. Hybridization and deep coalescence are additional processes, which may prevent representatives from the same biological species from forming monophyletic clades in phylogenetic analyses. In practice, we recognize species that are morphologically distinct and/or which form a monophyletic group in phylogenetic analysis of one or several loci, which is not contradicted by supported monophyletic groupings in phylogenetic results of any other locus.
We obtained data from the ITS, two nuclear coding genes and two variable regions of the mitochondrial SSU to investigate species limits within H. subsect. Denudata. The goal was to obtain data from all DNA regions for all collections on which species descriptions were based. Taxonomic types were not only included in the descriptions, but as far as possible also in the sequence analyses in order to link molecular results to traditional taxonomy.
Morphological methods have been refined. A large number of morphological characters have been parameterised in order to allow a relatively complete description and easy comparison between collections. These morphological characters span both macroscopic and microscopic detail. We have made extensive use of the work of Vesterholt (2005) and studies cited therein who adopted a classification to describe spore characters and formalised cheilocystidia descriptions. For the cheilocystidia we have further refined their classification to better describe their shape.
Most of the material used by Aanen and co-workers for their ICG studies has kindly been made available by Th.W. Kuyper. Thus, we are now able not only to recognize taxon clusters, but also to relate them to existing species and, thanks to the previous work of Aanen and others, to relate them to ICGs. The rigorous statistical approach of analysing numerous collections and their characters has also helped in the recognition and understanding of ecological preferences.
Within H. subsect. Denudata we include fifteen species occurring in Europe, eight of which are new to science. Hebeloma crustuliniforme was described in a recent publication (Vesterholt et al. 2014); this paper provides descriptions of the remaining fourteen taxa encountered in Europe and a key to all fifteen species, allowing the reader to appreciate species distinctions. Eleven of the fifteen species are likely to correspond to eleven ICGs identified by Aanen and Kuyper (Aanen 1999, Aanen & Kuyper 1999, 2004). As discussed above three ICGs relate to species outside H. sect. Denudata and the remaining ICGs relate to species in other subsections of H. sect. Denudata and will be addressed more fully in Eberhardt et al. and Grilli et al. (in prep.). We demonstrate that within this subsection – in spite of occasional problems in morphologically distinguishing between two species, rare mismatches between morphological and molecular evidence and gaps in the molecular data matrix when older specimen could not be sequenced for certain loci – that there is good overall consistency between morphological, molecular and seemingly also biological species circumscriptions.
Implications of earlier taxonomic decisions (Aanen 1999, Aanen & Kuyper 2004) with regard to the H. crustuliniforme complex and results based on environmental sequencing studies (reviewed by Timling & Taylor 2012) supported the view that agarics, including Hebeloma spp., of the arctic or alpine regions are essentially the same as temperate taxa at the species level. According to the results presented here for the H. crustuliniforme complex and H. subsect. Denudata, this assumption is debatable. Though taxa of both biomes are doubtlessly closely related, qualitative and quantitative differences can be observed between the arctic/alpine flora as opposed to floras of lower latitudes and altitudes.
MATERIALS AND METHODS
The majority of collections cited in species descriptions and used for molecular studies are included in the private herbarium of H.J. Beker. Some collections were obtained from other collectors and their private collections. Additional collections were obtained from public herbaria. The latter are referred to by their acronym, followed by a gap and a collection number. Private collection or collection numbers do not include gaps. Collections not contained in the herbarium of H.J. Beker are also referred to by their HJB database record number. This database contains all data pertaining to the collections and is intended for publication at a later stage.
Molecular analyses
Sequence data were obtained of five different DNA regions, ITS, RPB2, MCM7 (a DNA replication licensing factor) and the variable regions V6 and V9 of the mitochondrial SSU r-DNA. Not all data could be obtained for all collections; for some collections, mostly older collections, none or only partial ITS sequences could be obtained. Sequences were submitted to GenBank with the accession numbers KM390027–KM390104, KM390107–KM390759, KM390763–KM390775 (newly obtained for this study) and AY312982, JN943848–JN943881, KF309396–KF309406 and KF309426–KF309498.
Details of DNA extraction, PCR and sequencing primers have been provided earlier (Eberhardt et al. 2009, 2013, Eberhardt & Beker 2010, Vesterholt et al. 2014). Raw sequence data were edited in Sequencher (v. 4.9, Gene Codes Corporation, Ann Arbor, MI, USA). Ambiguous base calls were regularly encountered in sequences from nuclear ribosomal and protein-coding loci. Length deviant ITS copies within the same amplicon were treated as described in Eberhardt et al. (2013). In these cases the attempt was made to segregate the two constituent sequences, presumably representing different nuclei (Aanen et al. 2001), separately. Sequences with more than one indel were treated under the assumption that the two most likely constituent sequences were the two most similar ones, i.e. minimizing the number of assumed base exchanges. For analyses of concatenated alignments, the intragenomic consensus with the least number of ambiguous positions was used.
Sequence alignments were done in Mafft v. 7 (Katoh & Standley 2013) as implemented on http://mafft.cbrc.jp/alignment/software/, using the FFT-NS-i option for coding genes and the ITS and E-INS-i option for the variable mitochondrial SSU regions. Gap recoding following Simmons & Ochoterena (2000) was done using FastGap v. 1.2 (Borchsenius 2009) for the V6 and V9 sequence alignments. PartitionFinder (Lanfear et al. 2012) in combination with RAxML (v. 7.2.8-alpha, Stamatakis 2006) was used to determine the most efficient partitioning scheme for protein coding data and concatenated alignments. Concatenation of alignments was done in SequenceMatrix (Vaidya et al. 2011), using only one sequence per collection and locus, i.e. the consensus sequence in case of heterokaryotic data. Prior to the concatenation of different datasets, their compatibility was tested following the principle of Kauff & Lutzoni (2002), assuming a conflict to be significant if two different relationships for the same set of taxa, one being monophyletic and the other non-monophyletic, are supported by bootstrap with more than 70 % in ML analyses.
ML analyses for the compatibility test were done with RAxML v. 7.2.8-alpha on a local computer or RAxML-HPC BlackBox (v. 7.6.3) (Stamatakis 2006, Stamatakis et al. 2008) through the CIPRES Science Gateway (Miller et al. 2010). Maximum likelihood searches for tree building were carried out locally with 100 replicates using the GTRGamma model, selecting the best solution for each analysis. Fast Bootstrap searches were done locally or on the CIPRES server, with 1 000 replicates. Trees were visualized using FigTree v. 1.4.0 (Rambaut 2012). The assignment of collections and sequences to species follows morphology.
Distance values of ITS sequences were calculated in Mesquite (v. 2.75, Maddison & Maddison 2011, http://mesquiteproject.org) as ‘uncorrected p’ distances based on ambiguity differences, discounting gaps, and on the same alignment, that was also used for concatenation, considering the spacer regions and the 5.8 S rRNA (650 bp).
Morphological analysis
Morphological analysis is carried out with the help of a Biolomics-based database system (v. 7, http://www.bio-aware.com). Except where stated otherwise, all descriptions and measurements given are based on a number of collections. Wherever possible all macroscopic information is collected through the input of parametric data, either directly to the database or via the use of a standardised form. Where this is not possible, for example for old material and often for type material, original descriptions, and where possible photographs, are used to assemble this macroscopic information. All microscopic analysis was carried out on dried material and unless stated otherwise measurements given are average measurements.
For each collection at least 50 spores were measured in Melzer’s reagent, excluding the apiculus. The maximum length and width of each spore was measured and its Q value (ratio of length to width) calculated. Average length, width and Q value were calculated and recorded alongside the median, standard deviation and 5 % and 95 % percentiles. Additional measurements (not included in any of our keys) included spore area, circumference and colour.
The assessment of spore characters follows Vesterholt (2005): spore ornamentation on a scale from O0 (smooth) to O4 (ornamentation fairly strong, and always visible without immersion), the loosening of the perispore P0 (not loosening) to P3 (strongly and constantly loosening) and the dextrinoidity of the spores in Melzer’s reagent from D0 (indextrinoid) to D4 (strongly dextrinoid, immediately becoming dark brick to dark reddish brown).
The average width of the cheilocystidium apex appears to be an important character in the separation of species within Hebeloma (Vesterholt 2005). It is also important, when determining the average apex width, not to be selective with regard to the cystidia chosen for measurement. To determine the average width at the apex about 100 cheilocystidia were measured. These were measured while still on the lamella edge and by measuring all cystidia where the apex could be properly focused and measured. For other measurements, at least 20 cheilocystidia, separated from the lamella edge, were measured from each collection. Because of the complex shapes of the cheilocystidia four measurements were made: length, width at apex (A), width at narrowest point in central region (M) and maximum width in lower half (B). The measurements are given in this order and an average value was calculated for each of these measurements. Further, the ratios A/M, A/B and B/M are calculated for each cystidium and then these too are averaged. The measurements were made in 5 % KOH.
All microscopic measurements are made using a Leica DMRXA2 microscope system with a Leica DC300 camera connected to a computer running Leica IM1000 image capture software and Leica QWin image analysis software. Photographs of all relevant characters are taken and then all measurements and analysis is carried out on the computer system using the image analysis software, fed into spread sheets, where statistics are calculated, and then automatically transferred into the database. An advantage of this approach is that all measurements are repeatable and all information relating to these measurements, including all photographs, is stored with the rest of the collection information in the database.
Keys were built on the database as a set of complex queries. More than 1 250 collections of H. sect. Denudata amid more than 4 000 collections of Hebeloma, including their collection details, ecology and, for more than half these collections, morphometric parameters and results have been entered into the database. By having all data fully parameterised, collections can be compared with ease and database queries can be used to isolate those collections with similar features. This in turn enables keys to sections and to species to be built and continually be tested across a large number of collections. Principal component analyses were done in R (R Core Team 2014, Vu 2011).
RESULTS
All collections cited in the species descriptions of this paper were included in the molecular studies. The minimum goal was to obtain ITS sequences. For known species, collections were selected from a wide variety of habitats throughout Europe and in some cases also from other continents. We were able to locate and obtain type material for each of these taxa, as well as type material for a number of species that we believed were related to these taxa and might belong to this subsection, and this was also included in our studies. In total, ITS sequence data were obtained for over 550 collections and 271 collections were subjected to further and more detailed analysis. Hebeloma mediorufum was used as outgroup. This taxon, up to now only known from New Zealand, is, according to all DNA regions included in the analyses and the study of Rees et al. (2013), the closest relative of all known European members of H. sect. Denudata, without being inside the clade representing the European species of H. sect. Denudata.
No molecular data could be obtained for the lectotype of H. helodes (G 00053920; database record HJB1000054) and the holotype of H. eburneum (MPU GM1122; database record HJB1000095). The same applies to one collection used in the description of H. aanenii (collected by G. Bresadola, S F14406, database record HJB13470); H. eburneum (collected by G. Malencon, MPU GM1415, database record HJB12229); and for H. pusillum (collected by D. Aanen WBS 9648, database record HJB12518), though for the latter a V6 sequence could be obtained. For H. pusillum GLM GL42941 (database record HJB10993) the ITS sequence published by Boyle et al. (2006), AY312982, was used. In two cases only partial ITS sequences could be obtained: for the lectotype collections of H. alpinum (G GK13674; database record HJB1000060) only the ITS1; for the isotype of H. lutense (L 0054088, database record HJB1000011) only the ITS2; the ITS sequence of the holotype of H. lutense (P 59.232, database record HJB1000253) is complete.
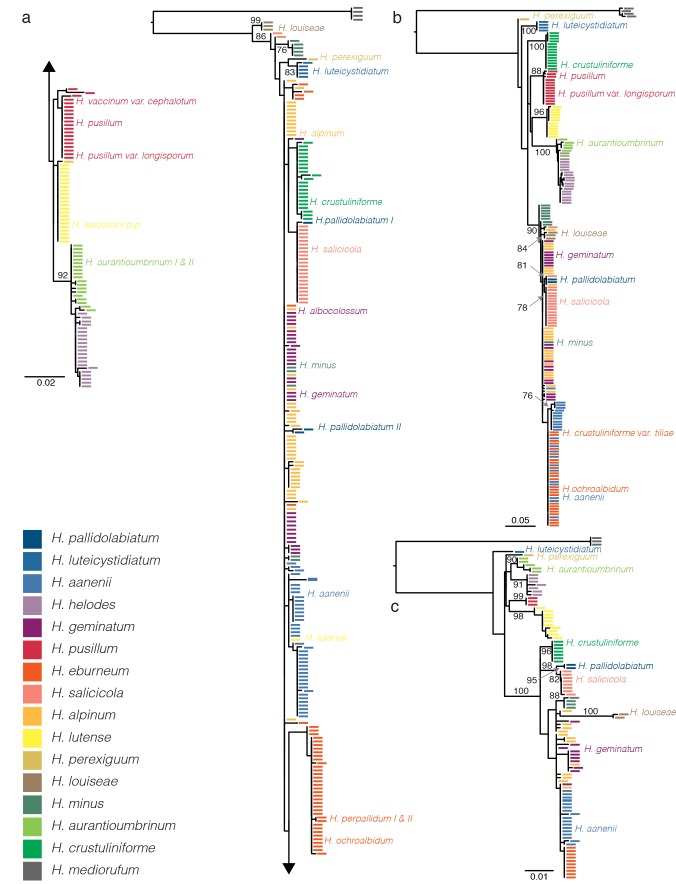
Table 1 summarizes the minima of the interspecific distances and maxima of the intraspecific distances of ITS – barcode – sequences. If the former value is lower than the latter value, correct species identification solely based on sequence similarity of the ITS barcode is bound to fail at least in some cases. Single locus alignments and trees for all DNA regions used were submitted to TreeBase (http://purl.org/phylo/treebase/phylows/study/TB2:S15738). Fig. 1 and 2 show 5 representations of the phylograms, colour-coded by species, with the position of type collections indicated at the side of the tree for loci for which sequence data could be obtained.
Table 1.
Inter-and intraspecific ‘uncorrected p’ distances of ITS-sequences (alignment 650 bp). Min(inter) – minimum of the interspecific distances; Max(intra) – maximum of the intraspecific distances; no. – number of collections considered; H. – Hebeloma.
| Species | No. | Min(inter) | Max(intra) |
|---|---|---|---|
| H. aanenii | 35 | 0 | 1.1 |
| H. alpinum | 34 | 0 | 0.2 |
| H. aurantioumbrinum | 12 | 0 | 0.3 |
| H. crustuliniforme | 20 | 0.2 | 0.2 |
| H. eburneum | 35 | 0 | 1.2 |
| H. geminatum | 26 | 0 | 0.3 |
| H. helodes | 21 | 0 | 0.5 |
| H. louiseae | 3 | 0.5 | 0 |
| H. luteicystidiatum | 4 | 0.2 | 0 |
| H. lutense | 23 | 0.2 | 0 |
| H. mediorufum | 4 | 3.4 | 0 |
| H. minus | 10 | 0 | 1.0 |
| H. pallidolabiatum | 2 | 0 | 0.2 |
| H. perexiguum | 1 | 0.3 | n.a. |
| H. pusillum | 36 | 0.6 | 1.1 |
| H. salicicola | 21 | 0 | 0.2 |
Fig. 1.
Best of 100 ML results of European Hebeloma subsect. Denudata of single gene analyses with 1 000 bootstrap replicates and H. mediorufum as outgroup. Only bootstrap values of ≥ 75 % are given. Species are colour coded (see legend). H. – Hebeloma. The placement of type collections is indicated by the name of the respective species at the side of the clade; the colour of the font matches the current taxonomic placement of the species. a. ITS; b. V6 region of the mitSSU; c. RPB2. For the ITS, length variant copies of the ITS from the same specimen were both included in the analysis and indicated by ‘I’ and ‘II’.
Fig. 2.
Best of 100 ML results of Hebeloma subsect. Denudata of single gene analyses with 1 000 bootstrap replicates and H. mediorufum as outgroup. Only bootstrap values of ≥ 75 % are given. Species are colour coded (see legend Fig. 1). H. – Hebeloma. The placement of type collections is indicated by the name of the respective species at the side of the clade; the colour of the font matches the current taxonomic placement of the species. a. V9 region of the mitSSU; b. MCM7.
In total, 314 ITS sequences represent 271 collections, because length deviant ITS copies retrieved from single collections were entered separately to see whether they would be part of different clusters supported by bootstrap of ≥ 75 %. This was not the case, so that for the concatenated alignment a single consensus sequence represents the 43 collections concerned (see below).
The ML result of the ITS analysis (Fig. 1a) shows only two species (H. louiseae and H. luteicystidiatum) are supported by boot-strap of ≥ 75 %. The clade, which contains the majority of H. minus ITS sequences, though supported, does not include all sequences from this species. Some clades are constituted predominantly by sequences of a single taxon (H. aanenii, H. auran-tioumbrinum, H. eburneum, H. helodes, H. lutense, H. pusillum and H. salicicola). The placement of the isotype of H. lutense in the middle of the main H. aanenii cluster is due to missing data; complete sequences of H. lutense (also the sequence of the holotype) differ mostly in the ITS1 from H. aanenii sequences. The obtained ITS2 sequence of the isotype shows no differences from any of the H. lutense sequences.
For the variable region V6 of the mitSSU, 190 sequences from the same number of collections were obtained. The alignment spans 408 DNA positions and 24 positions for recoded gap information. Bootstrap support is better with this region, with the species clades of H. crustuliniforme, H. louiseae, H. luteicystidiatum, H. lutense, H. pallidolabiatum and H. pusillum receiving bootstrap support of 86 % or more. Part of the H. crustuliniforme complex (Vesterholt et al. 2014), without H. crustuliniforme but including H. louiseae and H. pallidolabiatum (which were not included in the former publication) receive 90 % bootstrap support. There are additional supported clades (Fig. 1b), but neither of these represent species clades in the sense that all sequences of a given taxon are included.
For the other variable region of the mitSSU analysed here (Fig. 2a), V9, 106 sequences from the same number of collections were analysed. The alignment consists of 502 DNA positions and 20 positions represent recoded gaps. Four species (H. crustuliniforme, H. luteicystidiatum, H. lutense, H. pusillum) form bootstrap-supported species clades, the species clade of H. pallidolabiatum is unsupported.
Among the analysed loci, RPB2 (103 sequences of 103 collections, alignment 702 bp) is the one that on its own supports the species concepts described below best. Nine species clades receive bootstrap support, among them clades of H. aurantioumbrinum and H. helodes, H. minus and H. salicicola in addition to species clades mentioned before. Hebeloma eburneum is monophyletic without support. Only a single sequence could be obtained for H. luteicystidiatum, so that we do not know about the clade support. As in V6, the clade of the H. crustuliniforme complex receives a high bootstrap support. Hebeloma aanenii, H. alpinum and H. geminatum are paraphyletic.
Sequences for MCM7 were difficult to obtain, but 120 sequences of the same number of collections could be obtained; alignment length was 667 bp. The analysis supports eight species with bootstrap. Hebeloma salicicola forms an unsupported species clade. Sequence variation is rather high within some species of the H. crustuliniforme complex (i.e. H. aanenii, H. minus, H. salicicola) and, judging from SNPs, also intragenomically. We assume that the intragenomic variation is the result of heterokaryocity (Aanen et al. 2001). In high quality reads there are not more than two superposed peaks in a position.
Table 2 summarizes bootstrap results of single locus analyses, which were used for testing compatibility of the results of single gene analyses prior to concatenation. Results of single locus analyses are incompatible only with regard to the placement of the clades of H. perexiguum and H. pallidolabiatum. The results of the two mitochondrial variable regions differ with view to the placement of the H. perexiguum sequence, but in one case (V6) the support is rather low with 73 %. Hebeloma pallidolabiatum is in a supported clade with H. louiseae and H. minus for MCM7, which is incompatible with the supported RPB2 clade of H. pallidolabiatum and H. salicicola or the V6 result, where H. pallidolabiatum forms a supported clade with one collection of H. alpinum and H. salicicola each. This in turn forms a potential incompatibility with the supported H. salicicola clade of the RPB2 result.
Table 2.
Clades supported by bootstrap support values higher than 70 % in single locus analyses. In bold are support values supporting clades incompatible between some of the single locus ML results. n.a. – not applicable. Clades consisting of sequences assigned to a single species, but not containing all sequences of the respective species, are not listed
| Clade | ITS | V6 of mitSSU | V9 of mitSSU | RPB2 | MCM7 |
|---|---|---|---|---|---|
| H. aurantioumbrinum | 90 | 97 | |||
| H. helodes | 91 | 100 | |||
| H. aurantioumbrinum & H. helodes | 89 | 100 | 90 | 70 | |
| H. luteicystidiatum | 93 | 100 | 99 | n.a. | 100 |
| Ingroup excluding H. luteicystidiatum & H. perexiguum | 73 | ||||
| H. lutense | 96 | 100 | 98 | 99 | |
| H. pusillum | 77 | 88 | 100 | 99 | 100 |
| H. perexiguum & H. pusillum | 91 | ||||
| H. crustuliniforme | 74 | 100 | 99 | 96 | 99 |
| H. crustuliniforme complex excluding H. crustuliniforme | 90 | ||||
| H. louiseae | 99 | 84 | 100 | 99 | |
| H. minus | 88 | ||||
| H. minus & H. louiseae | 77 | ||||
| H. louiseae & H. pallidolabiatum & H. minus | 78 | ||||
| H. salicicola | 82 | ||||
| H. salicicola excluding HJB13087 & HJB9072 | 78 | ||||
| H. pallidolabiatum | 95 | 97 | |||
| H. pallidolabiatum & HJB13087 (H. salicicola) & HJB12204 (H. alpinum) | 81 | ||||
| H. salicicola & H. pallidolabiatum | 98 | ||||
| HJB9072 (H. salicicola) & HJB11986 (H. alpinum) | 80 | ||||
| HJB12806 (H. aanenii) and HJB10290, HJB12537, HJB12996 & HJB12804 (H. eburneum) | 76 | ||||
| H. aanenii (excluding HJB10450) & H. eburneum | 81 |
In spite of these incompatibilities we decided to concatenate the single locus alignments to see whether species not receiving support or being paraphyletic in single locus analyses would form (supported) monophyla in concatenated analyses. All loci were included in the concatenation, as there was no indication that mitochondrial DNA data show a completely different evolutionary history from nuclear data. Fig. 3 shows the ML result including five loci and all collections (158) for which data were available for at least three of the five concatenated loci. If only collections were included with a full dataset of five sequenced loci, the resulting topology is very similar, but the bootstrap support is better. Only H. alpinum (paraphyletic) and H. minus do not receive support ≥ 75 % and all other species, apart from H. geminatum and H. aanenii receive bootstrap support of 90 % or more. With four genes out of five, the result differs from the depicted phylogram by supporting the H. geminatum clade with 78 % bootstrap and the H. aanenii clade with 77 % bootstrap. With a minimum of two genes out of five, H. aanenii, H. eburneum, H. geminatum, and H. minus, in addition to H. alpinum that is paraphyletic in all analyses, also become paraphyletic.
Fig. 3.

Best of 100 ML results based on five DNA regions (ITS, V6 and V9 of the mitSSU, partial RPB2 and MCM7), including all collections for which sequences of at least three of the loci were obtained. Hebeloma mediorufum is outgroup. Bootstrap analyses are based on 1 000 replicates; only support values of ≥ 75 % are shown. Type collections are in bold. ICG – Intercompatibility group (see Aanen & Kuyper 1999).
In the ML result based on a minimum of three loci, one collection is ‘misplaced’ with regard to its morphological species assignment, namely H. alpinum HJB11051. DNA extraction, PCR and sequencing were repeated, but several markers place the collections in H. geminatum. One collection, H. geminatum, HJB11545, is not included in the H. geminatum clade and thus implicitly in the paraphyletic part of the tree constituting H. alpinum. In this case, the V9 sequence of the collection is odd and differs from the rest of the H. geminatum V9 sequences (and all other taxa with long V9 sequences in the alignment) in a number of positions. It differs very clearly from H. alpinum V9 sequences, which are considerably shorter than H. geminatum V9 sequences.
In Fig. 3 the intercompatibility group (ICG) assignment of (Aanen & Kuyper 1999) is stated for all collections for which this information was available. The figure shows that there is full agreement between the ICG, taxon names based on morphology and the molecular results. Collection WBS 9605, compatible with two ICGs belongs to H. alpinum morphologically and also clusters with other collections of this taxon.
We have been able to construct queries on the database, based purely on morphological characters, which can select the collections of a single clade (or ICG) as demonstrated in the keys provided below, with the single exception of H. aaneni and H. geminatum where there is clear evidence for two distinct species but we have been unable, thus far, to find a morphological character or set of characters on which such separation can consistently be unambiguous. Table 3 summarizes the most important morphological properties and ICG assignment (following Aanen & Kuyper 1999) of the recognized species.
Table 3.
Summary of ecological preferences and the most important morphological characters of the 15 species of Hebeloma subsect. Denudata. A/M, A/B and B/M refer to the average apex (A), median (M) and B (basal) width of the cheilocystidia. ICG (intercompatibility group) numbers follow (Aanen & Kuyper 1999).
| species: Hebeloma | aanenii | alpinum | aurantioumbrinum | crustuliniforme | eburneum | geminatum | helodes | louiseae | luteicystidiatum | lutense | minus | pallidolabiatum | perexiguum | pusillum | salicicola |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| habitat | woodland; on a variety of soils with a variety of trees; rarely alpine | arctic; alpine; with Salix or Dryas | arctic; alpine; boreal with Salix | woodland; dunes; with various trees | woodland; dunes; with various trees | woodland; on a variety of soils with a variety of trees; rarely arctic/alpine | woodland; dunes; various trees usually wet soil | arctic, with Salix | woodland; withSalix wet soil | woodland; dunes; with Salix usually wet soil | alpine; arctic; subalpine withSalix | arctic; with Salix | arctic; with Salix | woodland; with Salix often wet soil | dunes; woodlands; on a variety of soils with Salix orPopulus; rarely arctic |
| cap colour | cream to yellow to buff to yellow brown in centre; margin paler | cream to buff, sometimes centre Isabella or yellow brown | from yellow brown to umber but with some orange | cream to buff | pale, cream to yellowish | usually pale, cream to yellow or pale buff | pale, white to cream centre sometimes darker buff or yellowish brown | clay buff to Isabella | zonate: centre honey to dark brown; margin paler to buff | zonate; centre: yellow brown to dark brick; margin: white to cream | Isabella or buff to brown olive or umber, sometimes paler margin | sepia to dark brick in the centre, thin pale margin | uniformly grey brown | zonate: centre cinnamon to sepia to dark brick; margin paler to cream | zonate: centre ochre to red brown, sepia or dark brick; margin clay pink to buff or Isabella |
| pileus diameter | 20–170 | 12–70 | < 21 | 20–135 | 20–133 | 27–120 | 13–38 | < 15 | < 15 | 15–58 | 9–31 | 12–21 | 7–25 | 11–25 | 10–48 |
| median stipe width | 3.5–13 | 3–13 | 2.0–3.1(–3.5) | (6–)7.0–20(–26) | (3–)6–29(–36) | 6.5–12.8 | 2.9–4.5 | 2–3(–3.5) | 1.0–2.5 | 3–11 | 1.0–8.0 | 2–3.5 | 2 | 1.5–3.5 | (1.5–)2–7 |
| stipe Q (5–95%) | 2.1–12.0 | 2.3–6.0 | 5.5–7.0 | 1.7–6.9 | 2.8–9.4 | 2.5–9.2 | 4.7–20 | 4.6–7.6 | 12–17.5 | 4.2–12.3 | 4.8–14 | 6.2–6.6 | 6.3 | 10.8–21.6 | 3.5–11.1 |
| slenderness | 1.4–20.6 | 1.2–8.6 | 5.3–14.7 | 0.8–12.6 | 3.1–15.6 | 1–12.8 | 5.4–44.5 | 4.8–12.9 | 19.8–65 | 4.1–23.2 | 6.6–28 | 6.4–6.9 | 8.3 | 8.5–64.1 | 2–23.6 |
| number of lamellae (L) | 60–110 | 40–72 | 26–39 | 60–100 | usually 70–110; but some colls. 40–60 | 65–100 | 37–54 | 30–38 | 21–26 | 32–58 | 30–34 | 30–33 | 24–26 | 20–38 | 30–50 |
| spore ornamentation | O2,O3 | O1,O2(O3) | O1,O2(O3) | (O1)O2,O3 | O2,O3 | O2,O3 | O2,O3 | O1,O2 | O1,O2 | (O1)O2,O3 | O2,O3 | O2 | O1 | (O1)O2,O3 | O2,O3 |
| spore perispore | (P0)P1,P2 | P0,P1 | P0,P1 | (P0)P1(P2) | (P0)P1(P2) | (P0)P1(P2) | (P0)P1,P2 | P0 | P0,P1(P2) | (P0)P1,P2 | P0,P1,P2 | P0 | P0 | (P0)P1,P2 | (P0)P1,P2 |
| spore dextrinoidity | D0,D1(D2) | D0,D1,D2 | D1,D2 | (D0)D1,D2 | D1,D2 | D0,D1 | D0,D1(D2) | D0,D1 | D1,D2 | D1,D2 | D1(D2) | (D0)D1,D2 | D1 | (D0)D1,D2 | D2,D3 |
| av spore length | 8.8–10.9 | 11.0–13.7 | 10.2–11.8 | 10.8–12.2 | (10.2–)10.9–13.7 | 9.8–10.8(–11.6) | 9.1–10.8 | 12.3–12.8 | 11.6–11.9 | 9.4–11.7 | 11.2–13.1 | 12.8–13.5 | 11.7 | 11.4–13.6 | 11.2–13.3 |
| av spore width | 5.2–6.3 | 6.1–7.7 | 6.2–6.8 | 6.1–6.7 | (5.5–)6.1–7.1 | 5.4–6.3 | 4.9–6.0 | 7.5–7.7 | 6.1–6.5 | 5.4–6.4 | 6.2–7.7 | 7.2–7.6 | 7.2 | 5.6–6.7 | 6.1–7.5 |
| av spore Q | 1.60–1.95 | 1.60–1.97 | 1.60–1.85 | 1.70–1.89 | (1.60–)1.69–2.06 | 1.65–1.93 | 1.61–2.02 | 1.63–1.66 | 1.80–1.95 | 1.63–1.91 | 1.61–1.86 | 1.69–1.88 | 1.63 | 1.91–2.22 | 1.64–2.00 |
| av cheilocystidium length (μm) | 39–82 | 40–71 | 45–61 | 42–63 | 45–71 | 50–72 | 44–63 | 49–59 | 50–62 | 42–56 | 40–55 | 58–59 | 54 | 41–70 | 46–63 |
| av cheilocystidium apex width | 6.2–9.0 | 6.8–9.8 | 7.2–8.4 | 6.5–7.9 | 8.0–10.4 | 8.0–10.4 | 8.3–11.4 | 9.0–9.9 | 8.8–9.9 | 6.8–7.9 | 8.8–10.4 | 8.6–9.0 | 8.5 | 8.0–10.0 | 7.9–10.7 |
| av cheilocystidium median width | 3.6–5.3 | 3.6–5.1 | 4.0–4.8 | 3.4–4.7 | 3.6–4.9 | 4.0–4.7 | 4.0–4.9 | 4.4–5.4 | 4.0–4.3 | 3.4–4.5 | (3.7–)4.2–5.1 | 5.1–5.4 | 4.8 | 3.8–4.8 | 3.8–5.0 |
| av cheilocystidium basal width | 3.5–5.8 | 3.2–6.1 | 4.3–5.4 | 3.5–5.0 | 3.7–5.1 | 3.7–5.0 | 3.3–5.4 | 4.8–5.6 | 4.0–4.9 | 3.5–4.8 | (3.0–)4.3–5.9 | 5.9–6.3 | 6.4 | 3.6–4.9 | 3.6–5.9 |
| A/M | 1.61–2.49 | 1.61–2.74 | 1.61–1.99 | 1.61–2.32 | 1.78–2.71 | 1.76–2.57 | 1.90–2.86 | 2.01–2.42 | 2.27–2.77 | 1.63–2.39 | 1.99–2.44 | 1.61–2.05 | 1.75 | 1.71–2.71 | 1.83–2.63 |
| A/B | 1.51–2.48 | 1.52–2.71 | 1.62–1.98 | 1.52–2.32 | 1.80–2.72 | 1.68–2.85 | 2.02–3.38 | 1.99–2.25 | 2.21–2.68 | 1.58–2.34 | 1.82–3.02 | 1.57–1.69 | 1.45 | 1.68–2.52 | 1.60–2.82 |
| B/M | 0.85–1.24 | 0.87–1.24 | 0.97–1.18 | 0.90–1.22 | 0.90–1.19 | 0.81–1.19 | 0.77–1.17 | 0.92–1.24 | 0.98–1.22 | 0.95–1.22 | 0.84–1.24 | 1.09–1.24 | 1.33 | 0.94–1.20 | 0.96–1.27 |
| epicutis thickness | 100–400 | 60–160 | < 100 | 150–350 | 80–120 | 100–200 | 100–135 | < 100 | 50–60 | 100–180 | 40–100 | 80 | < 30 | 40–80 | 85–150 |
| ICG | 2 | 4 | n.a. | 5 | 3 | 1 | 12 | n.a. | 6 | 9 | 7 | n.a. | n.a. | 8 | 14 |
Principal component analyses with three parameters (average spore area, cheilocystidia apex width and number of complete lamellae, Fig. 4a), and with two parameters (average spore area and cheilocystidia apex width, Fig. 4b) that are important for species identification were run for the four taxa that are most difficult to distinguish from each other morphologically (H. aanenii, H. crustuliniforme, H. eburneum and H. geminatum) and H. alpinum which is most difficult to distinguish from these four in molecular terms. This analysis shows that these parameters alone are sufficient to group many collections correctly to species.
Fig. 4.
PCA of Hebeloma aanenii (aan), H. alpinum (alp), H. crustuliniforme (cru), H. eburnuem (ebu) and H. geminatum (gem). a. Using average apex width (a), average spore area (s) and average number of complete lamellae (l); b. excluding H. alpinum, using average apex width (a) and average spore area (s).
TAXONOMY
Hebeloma section Denudata (Fr.) Sacc.
Type. Hebeloma crustuliniforme (Bull.) Quél. emend. Vesterh., U. Eberh. & Beker.
Hebeloma subsection Denudata
Within subsect. Denudata we recognise the following fifteen taxa:
H. aanenii, H. alpinum, H. aurantioumbrinum, H. crustuliniforme, H. eburneum, H. geminatum, H. helodes, H. louiseae, H. luteicystidiatum, H. lutense, H. minus, H. pallidolabiatum, H. perexiguum, H. pusillum and H. salicicola
Cortina absent; universal veil not observed; smell usually more or less radish-like but sometimes absent; the lamellae usually with clear droplets but occasionally absent, particularly in dry weather, but then often leaving brown or rusty stains on the lamellae. Spores amygdaloid, sometimes with a papilla, O1-3; P0-2; D0-2, occasionally with some spores up to D3, av size 9.1–13.7 × 4.9–7.7 μm, Qav 1.60–2.17. Cheilocystidia with a majority capitate-, clavate- or spathulate-stipitate; i.e. swollen at the apex but fairly cylindrical below this apical swollen area but sometimes with a few cheilocystidia a little swollen near the base (capitate-, clavate- or spathulate-lageniform), particularly in smaller, possibly less mature cheilocystidia; average length between 40 μm and 75 μm; av width dimensions (μm): 6.8 < apex A < 11.4; 3.4 < median M < 5.4; 3.2 < base B < 6.4. Ratios: A/M > 1.6; A/B > 1.45; B/M < 1.35.
Notes — The main morphological feature that distinguishes species of H. subsect. Denudata is the shape of the cheilocystidium, significantly swollen at the apex, constricted in the median part and little swollen, if at all, in the bottom half, except in smaller, possibly less mature cheilocystidia.
Below we give descriptions of all species of H. subsect. Denudata discussed in this paper, with the exception of H. crustuliniforme, which was described in Vesterholt et al. (2014). Although a number of these species have been treated extensively in the literature, our diagnosis may be narrower (based wholly on the collections cited in this paper) and hence we feel a full description is necessary in order to properly define the morphological species concepts.
Key to Hebeloma section Denudata subsection Denudata
We provide a separate key for arctic/alpine collections. While this inevitably means some repetition between the two keys we believe the practical advantages make it worthwhile.
Key to Hebeloma subsection Denudata
1. Alpine or arctic collection with Salix or Dryas . . . . . Key 1
1. Not alpine or arctic, whatever the association . . . . Key 2
Key 1 – Hebeloma subsection Denudata
1. Av L ≥ 60 and av spore length < 11 μm . . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . Denudata Key 2 - 11
1. Av L < 60 or av spore length ≥ 11 μm . . . . . . . . . . . . . . . 2
2. Av L ≥ 40, spore length ≥ 11 μm with distinct papilla . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . H. alpinum
2. Any of the above conditions not satisfied . . . . . . . . . . . 3
3. Spores O3 and D2 and not D1 . . . . . . . . . . H. salicicola
3. Spores not O3 or not D2 or D1 . . . . . . . . . . . . . . . . . . . . 4
4. Av width of cheilocystidia apex < 8.5 μm . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . H. aurantioumbrinum
4. Av width of cheilocystidia apex ≥ 8.5 μm . . . . . . . . . . . . 5
5. Cheilocystidium ratio A/B > 1.8 . . . . . . . . . . . . . . . . . . . 6
5. Cheilocystidium ratio A/B ≤ 1.8 . . . . . . . . . . . . . . . . . . . 7
6. Spores O1 or O2, few if any spores O3 . . . . . H. louiseae
6. A large number of spores O3 . . . . . . . . . . . . . . . H. minus
7. Majority of spores O1 and av spore length ≤ 12 μm . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . H. perexiguum
7. At least some spores at least O2 and av spore length > 12 μm . . . . . . . . . . . . . . . . . . . . . . . . H. pallidolabiatum
Key 2 – Hebeloma subsection Denudata
1. L < 60. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
1. L ≥ 60. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
2. Av width of cheilocystidia apex < 8 μm and many cheilocystidia sinuate . . . . . . . . . . . . . . . . . . . . . . . . . H. lutense
2. Av width of cheilocystidia apex ≥ 8 μm or cheilocystidia not sinuate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3
3. Av spore length < 11 μm . . . . . . . . . . . . . . . . . . . . . . . . . 4
3. Av spore length ≥ 11 μm . . . . . . . . . . . . . . . . . . . . . . . . . 5
4. Cheilocystidia without consistent and distinct apical thickening . . . . . . . . . . . . . . . . . . . . . . . . . . H. aurantioumbrinum
4. Cheilocystidia with consistent and distinct apical thickening . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . H. helodes
5. Pileus almost uniformly coloured and pale (usually 3A2 or 4A2) and av stipe width in centre ≥ 6 mm . H. eburneum
5. Pileus distinctly 2-coloured (with brown centre) and av stipe width in centre ≤ 7 mm. . . . . . . . . . . . . . . . . . . . . . . . . . 6
6. L < 30 and cheilocystidia have a thick apical wall often appearing yellow under the microscope . . . . . . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . H. luteicystidiatum
6. L > 30 or cheilocystidia without such an apical wall . . . .7
7. Av stipe Q < 12, spore dextrinoidity at least D2 . . . . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . H. salicicola
7. Av stipe Q ≥ 12 or many spores at most D1 . . . . . . . . . 8
8. Av spore Q < 1.9 . . . . . . . . . . . . . . . . . . . . . . . . . H. minus
8. Av spore Q ≥ 1.9 . . . . . . . . . . . . . . . . . . . . . . . H. pusillum
9. Av spore width is ≥ 6.4 μm or av, spore length > 10.75 μm and av spore width > 6 μm . . . . . . . . . . . . . . . . . . . . . . 10
9. Av spore width < 6.4 μm and av, spore length ≤ 10.75 μm or av spore width ≤ 6 μm . . . . . . . . . . . . . . . . . . . . . . . 11
10. Av width of cheilocystidia apex ≥ 8 μm . . . . H. eburneum
10. Av width of cheilocystidia apex < 8 μm H. crustuliniforme
11 . Av width of cheilocystidia apex > 9 μm . . . . H. geminatum
11.Av width of cheilocystidia apex ≥ 8 μm and ≤ 9 μm . . . . .. . . . . . . . . . . . . . . . . . . . . . . . . H. aanenii/H. geminatum
11. Av width of cheilocystidia apex < 8 μm . . . . . . . H. aanenii
Hebeloma aanenii Beker, Vesterh. & U. Eberh., sp. nov. — MycoBank MB809905; Fig. 5a, 6, 7
Fig. 5.
Photo of: a. Hebeloma aanenii BR-MYCO 173987-66 (holotype); b. H. aurantioumbrinum BR-MYCO 173985-64 (holotype); c. H. geminatum C-F-90152 (JV96-341) (holotype); d. H. louiseae BR-MYCO 173982-61 (holotype); e. H. luteicystidiatum BR-MYCO 166233-72 (holotype); f. H. pallidolabiatum BR-MYCO 174908-17 (holotype); g. H. perexiguum BR-MYCO 173979-58 (holotype); h. H. salicicola BR-MYCO 173977-56 (holotype). — Photos: a, b, d, f–h. H. Beker; c. J. Vesterholt; e. P. Derboven.
Fig. 6.
Hebeloma aanenii (BR-MYCO 173987-66, holotype). a, b. Spores and spore ornamentation ×1 600 in 5 % KOH; c, d. spores and spore ornamentation ×1 600 in 5 % Melzer’s reagent; e. cheilocystidia ×1 000 in 5 % KOH; f. caulocystidia ×500 in 5 % KOH; g. basidia ×1 000 in 5 % KOH; h. epicutis hyphae ×500 in 5 % KOH. — Scale bars: 10 μm.
Fig. 7.
Hebeloma aanenii (BR-MYCO 173987-66, holotype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
Etymology. In honour of Duur Aanen to whom we are deeply indebted for carrying out extensive research on biological mating between Hebeloma spp. as well as molecular analysis.
Type. POLAND, Bialowieski Park Narodowytrup (N52.72111 E23.9056; alt. ± 160 m) on acid soil in mixed ancient woodland pathside with Carpinus betulus, Picea sp., Populus sp. and Quercus sp., 19 Sept. 2008, H. Beker, I. Kałucka, holotype BR BR-MYCO 173987-66; isotype C C-F-90147; database record HJB12630.
Diagnosis — Hebeloma aanenii has the typical cystidia of H. subsect. Denudata. It differs from H. alpinum, H. crustuliniforme, H. eburneum by the average size of its spores (length < 11 μm and width < 6 μm) and normally from H. geminatum by the average width of the cheilocystidium apex which is always < 9 μm and usually < 8 μm and from the rest of the species of its subsection by the average number of lamellae, which is always ≥ 60. It can be differentiated from H. geminatum based on sequence comparison of the partial RPB2 sequences and usually also the internal transcribed spacer of the nuclear ribosomal genes.
Basidiomes usually in scattered groups, sometimes caespitose, sometimes growing gregariously, sometimes solitary. Pileus from 18–170 mm diam, convex, often umbonate; surface often very viscid in appearance, tacky when moist never hygrophanous but occasionally spotted; cuticle colour usually from cream to buff and sometimes yellowish even ochraceous, yellow brown or clay-coloured in the centre but then becoming paler towards the margin which is always very pale at most light buff; pileus margin often involute, particularly in young specimens, sometimes straight but can be serrate, scalloped or crenulate. Lamellae emarginate to adnate, crowded (L = 60–110) with a maximum depth of 2.5–9 mm; colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, paler than lamella surface; droplets on the lamella edge are usually present and visible to the naked eye; lamellules frequent. Stipe central, cylindrical often clavate and occasionally bulbous, (22–)25–108(–130) × 3.5–9(–13) mm and up to 20 mm at the base; white or alutaceous, sometimes but not usually discolouring from the base when handled or with age; surface dry, usually strongly floccose particularly towards the apex but at least pruinose; interior stuffed when young but sometimes becoming hollow with age and sometimes with a superior wick. Cortina not observed. Flesh rather thick, cream or pale brown. Smell raphanoid. Taste bitter. Spore deposit brownish olive to greyish brown or umber.
Spores amygdaloid, with small apiculus and rounded at the end opposite the apiculus, with a distinct thinning of the spore wall but rarely with any papilla, guttulate with one or more oily drops, weakly to distinctly ornamented, sometimes with some sign of loosening perispore in a few spores and weakly dextrinoid (O2,O3; (P0)P1,P2; D0,D1(D2)); spore colour under the microscope yellow-brown to brown; spore size based on n = 109 spores of the holotype, 5–95 % percentile range 8.8–11.3 × 5.1–5.9 μm, with median 10.0 × 5.4 μm and av 10.0 × 5.5 μm with SD length 0.73 μm and width 0.29 μm, Q value 5–95 % percentile range 1.66–2.01, with median 1.85 and av 1.83 with SD 0.11; spore size based on 36 collections medians 8.7–11.0 × 5.2–6.2 μm and av 8.8–10.9 × 5.2–6.3 μm with SD length 0.42–1.00 μm and width 0.18–0.65 μm, Qav 1.60–1.95. Basidia cylindrical to clavate and 4-spored, 21–43 × 6.1–10.2 μm, with av 22–36 × 6.6–9.1 μm. Pleurocystidia not found. Cheilocystidia usually clavate-stipitate and sometimes spathulate-stipitate, occasionally slightly swollen towards the base (clavate-lageniform) and occasionally with some apical or median thickening, septate, sinuate and occasionally bifurcate; width of apex holotype 5–95 % percentile range 5.5–9.0 μm, with median 7.3 μm and av 7.2 μm with SD 1.01 μm; across 36 collections median 6.2–9.0 μm and av 6.2–9.0 μm (of the 36 collections almost 80 % have the cheilocystidium apex av ≤ 8 μm); with n ≥ 20 selected cheilocystidia of 36 collections the 5–95 % percentile ranges are 28–104 × 4.6–12.6 × 2.4–7.4 × 2.3–9.0 μm while the averages are 39–82 × 6.2–9.0 × 3.6–5.3 × 3.5–5.8 μm and 61 × 7.2 × 3.7 × 3.9 μm av for the holotype. The av cheilocystidia ratios for the 36 collections were: A/M = 1.61–2.49; A/B = 1.51–2.48; B/M = 0.85–1.24. Caulocystidia resemble cheilocystidia but are often more clavate-lageniform shaped, up to 100 μm long and 11 μm wide at the apex. Pileipellis is an ixocutis with a very thick epicutis 100–400 μm, embedded hyphae up to 5–7 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis yellowish and made up of cylindrical to isodiametric elements. Subcutis contains isodiametric elements and the trama below the subcutis contains cylindrical, ellipsoid and thick sausage shaped elements up to 20 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — Hebeloma aanenii appears to be widespread across Europe, although we do not have confirmed records from south-west Europe. It appears to grow in a variety of habitats, on both acid and calcareous soils, often on woodland pathsides and also in alpine areas. It appears to form mycorrhiza with a variety of trees. Records include Abies, Betula, Carpinus, Dryas, Eucalyptus, Fagus, Helianthemum, Picea, Pinus, Populus, Quercus, Salix and Tilia. We have one confirmed collection of H. aanenii with Salix sp. in New Zealand.
Additional specimens examined. BELGIUM, prov. Luxembourg, Barvaux (c. N50.34 E5.49, alt. c. 200 m) on wet soil in mixed woodland under Salix sp., 18 Oct. 2003, E. Emmett, HJB8896; prov. Luxembourg, Wilbauroche (N49.81335 E5.27330, alt. c. 312 m) on rotten litter in broadleaf woodland under Corylus sp., Fagus sp., 17 Sept. 2004, H. Beker HJB10607; prov. Luxembourg, Goutelle (N50.01500 E5.2291667, alt. c. 401 m) on grassy soil in mixed woodland under Picea sp., 3 Oct. 2004, M. Ghyselinck HJB10164; prov. Namur, Biron (c. N50.30 E5.11, alt. c. 250 m) on rotten litter in conifer woodland under various broadleaf trees, 7 Oct. 2004, M. Lenne HJB10282; prov. Brussels, Scheutbos (N50.8531667 E4.2950500, alt. c. 58 m) on grassy soil in broadleaf woodland under Betula sp., Salix sp., 28 Oct. 2007, H. Beker HJB12164. – CZECH REPUBLIC, Moravia, LPA Moravian Karst, NNR Vyvery Punkvy (N49.37290 E16.72823, alt. c. 487 m) on soil in mixed woodland under Fagus sylvatica, 10 Oct. 2008, V. Antonin HJB12717; Moravia, LPA Moravian Karst, NNR Vyvery Punkvy (N49.37245 E16.73003, alt. c. 487 m) on grassy soil in mixed woodland scrub under Populus sp., 10 Oct. 2008, S. Kelly HJB12721. – DENMARK, Region EJ, Århus, Brabrand, Gellerupparken, Dortesvej (N56.16041 E10.13308, alt. c. 50 m) on clayey soil in urban habitat under Populus tremula, 27 Oct. 2000, J. Vesterholt HJB3733. – ENGLAND, Mid Lancashire, Gait Barrows (N54.19061 W2.801503, alt. c. 31 m) on rotten litter in woodland under Betula sp., 25 Sept. 2002, H. Beker HJB9329; Warwick, Bishopton (N52.19831 W1.74099, alt. c. 64 m) on grassy soil under Tilia sp., 5 Oct. 2004, A. Brand HJB11219; Buckinghamshire, Rushbeds (c. N51.830 W1.030, alt. c. 100 m) on rotten litter in woodland under Quercus sp., Salix sp., 20 Oct. 2004, D. Schafer HJB10441; Derby, Ladybower (N53.38768 W1.754891, alt. c. 208 m) on rotten litter in broadleaf woodland under Betula sp., 24 Oct. 2004, P. Ardron HJB10435; Derby, Ladybower (N53.38768 W1.754891, alt. c. 208 m) on rotten litter in broadleaf woodland under Betula sp., Fagus sp., Pinus sp., Salix sp., 25 Oct. 2004, H. Beker HJB10446; Derby, Ladybower (N53.38768 W1.754891, alt. c. 208 m) on rotten Litter in broadleaf woodland under Betula sp., Fagus sp., Pinus sp., Salix sp., 25 Oct. 2004, H. Beker HJB10450; Nottinghamshire, Clumber Park (N52.73547 W1.096525, alt. c. 64 m) on grassy soil in broadleaf woodland under Salix sp., 30 Oct. 2004, C. Hobart HJB10696; Warwick, Bishopton (N52.19831 W1.74099, alt. c. 81 m) under Populus trichocarpa, 1 Nov. 2004, A. Brand HJB11220; Nottinghamshire, Daneshill Energy Forest near Suttin Cum Lound SK6785 (N53.36553 W0.98410, alt. c. 11 m) on grassy, mossy, sandy soil in woodland plantation under Eucalyptus sp., 13 Nov. 2010, H. Beker, C. Hobart HJB13734; Derby, Ladybower (N53.38768 W1.754891, alt. c. 208 m) on rotten litter in broadleaf woodland under Betula sp., Fagus sp., Pinus sp., Salix sp., 25 Oct. 2004, H. Beker HJB10452. – FRANCE, Hautes-Pyrenees, las gabietons, Gavarnie (c. N42.695 W0.0320, alt. c. 2550 m) under Salix herbacea, Salix retusa, 14 Sept. 2005, G. Corriol GC05 09 14 06, HJB12954; Nord Pas de Calais, Abscon (N50.32365 E3.29990, alt. c. 60 m) on rotten litter in chalk quarry site under Quercus sp., 13 Nov. 2006, C. Lecuru HJB11784; Nord Pas de Calais, Abscon (N50.32365 E3.29990, alt. c. 60 m) on mossy soil in chalk quarry site under Quercus sp., 13 Nov. 2006, C. Lecuru HJB11785; Aude, Le Bois du Pinet (N42.85661 E1.97465, alt. c. 840 m) on soil in mixed woodland under Salix sp., 13 Oct. 2008, P. Roux HJB12729. – ITALY, Trentino, Goccadioro (c. N46.05540 E11.13720, alt. c. 245 m) under broadleaf trees, Nov. 1914, G. Bresadola S F14406, database record HJB13470. – NETHERLANDS, Flevoland, Lelystad, Oostvaarderplassen (c. N52.450 E5.3667, alt. c. 0 m) under Salix sp., 11 Oct. 1995, D. Aanen WBS 9570, database record HJB12805; Groningen, Eemshaven (c. N53.455 E6.806, alt. c. 0 m) under Salix repens, 3 Oct. 1996, D. Aanen WBS 9677, database record HJB12478; Groningen, Eemshaven (c. N53.455 E6.806, alt. c. 0 m) under Salix repens, 3 Oct. 1996, D. Aanen WBS 9671, database record HJB12806; Utrecht, Lunetten (c. N52.03453 E5.07562, alt. c. 0 m) under Salix alba, 13 Oct. 1996, D. Aanen WBS 9683, database record HJB12475; Utrecht, Lunetten (c. N52.03453 E5.07562, alt. c. 0 m) under Salix alba, 13 Oct. 1996, D. Aanen WBS 9684, database record HJB12476; Utrecht, Lunetten (c. N52.03453 E5.07620, alt. c. 0 m) under Salix alba, 13 Oct. 1996, K. Wolfs, D. Aanen WBS 9685, database record HJB12477. – NEW ZEALAND, Nelson, Stephens Bay (c. S41.050 E173.010, alt. c. 20 m) on soil in riverside scrub under Salix sp., 3 May 2004, P. Leonard PL13504, PDD 102994, HJB10692. – NORWAY, Tislet (c. N61.083 E6.500, alt. c. 240 m) in broadleaf woodland pathside under Betula sp., 5 Aug. 2004, A. Andrews HJB10763. – POLAND, Mt Kamiensk (the outer damping ground of the Belchatow Lignite Mine), forest distr. 297 (by the pond) (N51.22134 E19.43924, alt. c. 340 m) on sandy soil in mixed woodland plantation under Picea abies; Salix sp., 23 Sept. 2008, I. Kałucka; H. Beker LOD, HJB12676. – SWEDEN, Uppland, Nåsten (c. N59.04 E18.65600, alt. c. 0 m) on grassy soil in mixed woodland, 20 Sept. 2003, A. Taylor AT2003063, HJB10670. – SWITZERLAND, Schiahorn, Strelapass, Davos (c. N46.822574 E9.781488, alt. c. 2200 m) under Dryas octopetala, Helianthemum sp., 8 Sept. 1996, D. Aanen WBS 9620, database record HJB12508; Mollendruz, Vaud (c. N46.649202 E6.367389, alt. c. 1200 m) under Abies sp., Fagus sp., Salix caprea, 21 Sept. 1996, D. Aanen WBS 9626, database record HJB12511.
Notes — The dominant cheilocystidial shape, clavate- to spathulate-stipitate clearly defines H. aanenii as belonging to H. subsect. Denudata. Hebeloma aanenii is a constituent of the H. crustuliniforme complex and most likely corresponds to ICG2 of Aanen & Kuyper (1999). It is likely that many collections of this species have been recorded under the name H. crustuliniforme and exist worldwide in herbaria under this name. It is morphologically most similar to H. crustuliniforme, H. eburneum, H. alpinum and H. geminatum. But its spores, normally < 11 μm long and < 6 μm wide distinguish it from the first three species. It can be distinguished from other members of this subsection by the number of complete lamellae, which is always ≥ 60. Until now we have found no consistent morphological character to unambiguously separate H. aanenii and H. geminatum. However, we can often separate these two taxa. The cheilocystidium average apex width for H. aanenii is usually smaller than that for H. geminatum. From our records, if the average width of the apex of the cheilocystidium is < 8 μm then the collection is almost certainly H. aanenii. However, the average apex width can reach 9 μm. Average apex widths in this interval between 8–9 μm can be found in either taxon. This is responsible for the overlap of the ellipses of H. aanenii and H. geminatum in the PCA diagram in Fig. 4b.
In spite of the rather large intraspecific ITS variation it should normally be possible to recognize H. aanenii based on this locus. Only a single collection (WBS 9620, database record HJB12508) out of 33 is not included in the H. aanenii clade. Based on three or five loci, H. aanenii is monophyletic and weakly supported (Fig. 3), including WBS 9620. In none of the single locus phylogenies do all H. aanenii sequences form a monophylum. The combination of V6 and V9 also unambiguously identifies H. aanenii, though neither locus on its own suffices.
Hebeloma alpinum (J. Favre) Bruchet, Bull. Mens. Soc. Linn. Lyon 39, 6 (Suppl.): 68. 1970. — MycoBank MB314944; Fig. 8, 9
Fig. 8.
Hebeloma alpinum (G GK13674, lectotype). a, b. Spores and spore ornamentation ×1 600 in 5 % Melzer’s reagent; c, d. spores and spore ornamentation ×1 600 in 5 % KOH; e, f. cheilocystidia ×1 000 in 5 % KOH; g. basidium ×1 000 in 5 % KOH; h. cheilocystidia ×500 in 5 % KOH; i. epicutis hyphae ×500 in 5 % KOH. — Scale bars: 10 μm.
Fig. 9.
Hebeloma alpinum (G GK13674, lectotype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
Type. SWITZERLAND, Val dal Botsch (c. N46.65 E10.10, alt. c. 2600 m) alpine scrub, on calcareous soil with Dryas octopetala and Salix herbacea, 27 Aug. 1949, J. Favre, lectotype G GK13674; database record HJB1000060, selected by Vesterholt in Symb. Bot. Upsal. 30 (no. 3): 134, 1995.
Basidiomes usually in scattered groups, sometimes solitary, rarely caespitose. Pileus 12–70 mm diam, convex often umbonate, tacky when moist but never hygrophanous; cuticle colour quite variable from cream through pinkish buff to Isabella and yellow brown, to clay buff, cinnamon or even sepia, often unicoloured but sometimes paler towards the margin which may be pinkish buff, clay buff or greyish buff through to cream or white; pileus margin often involute particularly in young basidiomes but often becoming serrate or crenulate in older basidiomes. Lamellae emarginate, usually moderately crowded (L = 40–72); maximum depth of 3–9 mm; colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, usually paler than lamella surface; droplets normally visible on the lamella edge usually by naked eye but sometimes absent; lamellules frequent. Stipe central, sometimes cylindrical but more often clavate, rarely bulbous, becoming hollow with age, 11–50 × 3–13 mm and up to 15 mm at the base with no (or very weak) discolouration towards the base of the stipe; surface dry, pruinose to floccose, especially towards the apex. Cortina not observed. Flesh thick, white to pale buff. Smell raphanoid, sometimes weak, sometimes absent and occasionally a hint of cocoa. Taste mild but sometimes a little bitter. Spore deposit brownish olive to clay-buff or Isabella. Exsiccata with no special features.
Spores amygdaloid or limoniform, with small apiculus and rounded at the end opposite the apiculus, with some thinning of the spore wall, and usually with a papilla, often very noticeable, sometimes guttulate with one or more oily drops, usually weakly ornamented but sometimes more distinctly ornamented, sometimes with slightly loosening perispore in a few spores and weakly but sometimes distinctly dextrinoid (O1,O2(O3); P0,P1; D0,D1,D2); spore colour under the microscope brown, sometimes with a yellow tinge; spore size based on n = 60 spores of the holotype, 5–95 % percentile range 11.2–13.3 × 6.6–8.0 μm, with median 12.3 × 7.3 μm and av 12.3 × 7.3 μm with SD length 0.65 μm and width 0.45 μm, Q value 5–95 % percentile range 1.50–1.86, with median 1.69 and av 1.69 with SD 0.11; spore size based on 34 collections medians 10.9–13.7 × 6.1–7.6 μm and av 11.0–13.7 × 6.1–7.7 μm with SD length 0.53–1.16 μm and width 0.25–0.57 μm, Qav 1.60–1.97. Basidia cylindrical to clavate and 4-spored, 25–45 × 7.1–11.7 μm, with av 28–40 × 8.0–11.1 μm and basidium Qav in the range 3.0–4.3. Pleurocystidia not found. Cheilocystidia clavate-stipitate or spathulate-stipitate, occasionally swollen towards the base (clavate-lageniform), sometimes with thickening of the apex or thickening of the median, sometimes septate, rarely bifurcate, sinuate or rostrate; width of apex holotype 5–95 % percentile range 6.4–9.7 μm, with median 7.8 μm and av 7.9 μm with SD 1.10 μm; across 34 collections median 6.8–9.8 μm and av 6.8–9.8 μm; with n ≥ 20 selected cheilocystidia of 34 collections the 5–95 % percentile ranges are 30–92 × 4.8–13.1 × 2.7–6.3 × 2.3–8.1 μm while the averages are 40–71 × 6.8–9.8 × 3.6–5.1 × 3.2–6.1 and 58 × 7.9 × 4.4 × 4.3 μm av for the holotype. The av cheilocystidia ratios for the 34 collections were: A/M = 1.61–2.74; A/B = 1.52–2.71; B/M = 0.87–1.24. Caulocystidia resemble cheilocystidia, up to 120 × 11 μm wide at the apex. Pileipellis is an ixocutis with an epicutis from relatively thin to medium thick, 60–160 μm, embedded hyphae up to 5–6 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Subcutis yellow to orange and made up of cylindrical to isodiametric elements. Trama below the subcutis contains angular, ellipsoid, cylindrical, spherical and sausage-shaped elements up to 18 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — Hebeloma alpinum has been recorded with a number of species of Salix in both arctic and alpine environments. It has also been recorded with Dryas octopetala when Salix was not recorded as present. We strongly suspect that it can be mycorrhizally associated with Dryas. We have no records of this taxon outside of such habitats. Our database records of H. alpinum have the following Salicaceae associations: Salix herbacea, S. polaris, S. reticulata and S. retusa. We have records in both acid and calcareous soils, bare, grassy and sandy. As well as the 34 collections upon which our description has been based, we have a number of other confirmed records of H. alpinum including a number from outside of Europe. So far we have no records on our database from alpine areas outside Europe but we suspect it may well be present in these regions too.
Additional specimens examined. GREENLAND, Zackenberg, just south of Teltdammen (c. N74.30 W21.00, alt. c. 700 m) in scrub under Dryas octopetala, Salix sp., 20 July 1999, T. Borgen TB99.023, duplicate HJB12194; Zackenberg, 100 m west of Zackenberg River (c. N74.50 W21.00, alt. c. 700 m) on dry soil in scrub under Dryas octopetala, 3 Aug. 1999, T. Borgen TB99.199, duplicate HJB12204. – ICELAND, Valavatn (N64.86655 E23.5597167, alt. c. 301 m) on bare soil in scrub under Salix herbacea, 29 July 2005, H. Beker, M.L. Beker HJB11051. – ITALY, Lac Verney (N45.688183 E6.882441, alt. c. 2090 m) under Salix reticulata, 24 Aug. 2009, G. Corriol HJB13096. – NORWAY, Møre & Romsdal, Beiaren kommune: Sokumfjellet (N66.87940 E14.28040, alt. c. 800 m) under Dryas octopetala, Salix polaris, Salix reticulata, 4 Aug. 2008, P. Larsen Larsen 56-2008, HJB13008. – SVALBARD, Endalen (N78.1937333 E15.7891167, alt. c. 29 m) on soil in grazed scrub under Salix polaris, 13 Aug. 2007, M.L. Beker HJB11986; Ekmanfjorden (N78.61705 E14.837183, alt. c. 9 m) on soil in maritime coastal scrub under Salix polaris, 15 Aug. 2007, H. Beker, M.L. Beker HJB11997; Ekmanfjorden (N78.617033 E14.837683, alt. c. 12 m) on soil in maritime coastal scrub under Salix polaris, 15 Aug. 2007, H. Beker, M.L. Beker HJB12000; Ekmanfjorden (N78.617033 E14.837683, alt. c. 12 m) on soil in maritime coastal scrub under Salix polaris, 15 Aug. 2007, H. Beker, M.L. Beker HJB12001; Ekmanfjorden (N78.617233 E14.838083, alt. c. 14 m) on soil in maritime coastal scrub under Salix polaris, 15 Aug. 2007, J. Sandmo HJB12002; Dicksonfjorden (N78.6207667 E14.8823000, alt. c. 24 m) on soil in maritime coastal scrub under Salix polaris, 15 Aug. 2007, H. Beker, M.L. Beker HJB12004; Dicksonfjorden (N78.6210333 E14.8840500, alt. c. 25 m) on soil in maritime coastal scrub under Salix polaris, 15 Aug. 2007, H. Beker, M.L. Beker HJB12005. – SWITZERLAND, Graubünden Samnaun (c. N46.940 E10.360, alt. c. 1900 m) under Salix reticulate, Salix retusa, 28 Aug. 1984, H. Knudsen C HK36-84, HJB10642; Adelboden, Bern (c. N46.500 E7.550, alt. c. 1900 m) on calcareous soil under Salix retusa, 5 Sept. 1996, D. Aanen WBS 9605, database record HJB12498; Adelboden, Bern (c. N46.500 E7.550, alt. c. 1900 m) on calcareous soil under Salix retusa, 5 Sept. 1996, D. Aanen WBS 9607, database record HJB12499; Adelboden, Bern (c. N46.500 E7.550, alt. c. 1900 m) on calcareous soil under Dryas octopetala, Salix retusa, 5 Sept. 1996, D. Aanen WBS 9609, database record HJB12500; Adelboden, Bern (c. N46.500 E7.550, alt. c. 1900 m) on calcareous soil under Dryas octopetala, Salix retusa, 5 Sept. 1996, D. Aanen WBS 9613, database record HJB12502; Adelboden, Bern (c. N46.500 E7.550, alt. c. 1900 m) on calcareous soil under Salix retusa, 5 Sept. 1996, D. Aanen WBS 9608, database record HJB12503; Adelboden, Bern (c. N46.500 E7.550, alt. c. 1900 m) on calcareous soil in mountain scrub under Dryas octopetala, Salix retusa, 5 Sept. 1996, D. Aanen WBS 9606, database record HJB12504; Adelboden, Bern (c. N46.500 E7.550, alt. c. 1900 m) on calcareous soil under Dryas octopetala, Salix sp., 5 Sept. 1996, D. Aanen WBS 9614, database record HJB12505; Adelboden, Bern (c. N46.500 E7.550, alt. c. 1900 m) on calcareous soil under Salix retusa, 5 Sept. 1996, D. Aanen WBS 9617, database record HJB12506; Adelboden, Bern (c. N46.500 E7.550, alt. c. 1900 m) on calcareous soil under Salix retusa, 5 Sept. 1996, D. Aanen WBS 9616, database record HJB12507; Adelboden, Bern (c. N46.500 E7.550, alt. c. 1900 m) on calcareous soil under Dryas octopetala, 5 Sept. 1996, D. Aanen WBS 9615, database record HJB12998; Spittelmatte (N46.440 E7.640, alt. c. 2000 m) on rotten litter in scrub under Dryas octopetala, 9 Aug. 2005, H. Beker, M.L. Beker HJB11085; Spittelmatte (N46.440 E7.640, alt. c. 1909 m) on herbaceous litter in scrub under Dryas octopetala, 9 Aug. 2005, H. Beker, M.L. Beker HJB11087; Spittelmatte (N46.4195 E7.62375, alt. c. 2220 m) on herbaceous litter in scrub under Dryas octopetala, 9 Aug. 2005, H. Beker, M.L. Beker HJB11088; Spittelmatte (N46.41050 E7.62220, alt. c. 2000 m) on bare soil in scrub under Salix sp., 9 Aug. 2005, H. Beker, M.L. Beker HJB11092; Spittelmatte (N46.41070 E7.62220, alt. c. 2000 m) on bare soil in scrub under Salix sp., 9 Aug. 2005, H. Beker, M.L. Beker HJB11094; Spittelmatte (N46.43953 E7.63781, alt. c. 2000 m) on rotten litter in scrub under Dryas octopetala; Salix sp., 9 Aug. 2005, H. Beker, M.L. Beker HJB11100; Corno Gries (N47.480 E8.500, alt. c. 2500 m) on grassy soil in scrub under Salix sp., 11 Aug. 2005, H. Beker, M.L. Beker HJB11117; Corno Gries (N46.4665833 E8.4069500, alt. c. 2500 m) on litter in scrub under Salix herbacea, 11 Aug. 2005, H. Beker, M.L. Beker HJB11123; Albulapass (N46.58200 E9.84300, alt. c. 2300 m) on bare soil in scrub under Salix sp., 12 Aug. 2005, H. Beker, M.L. Beker HJB11132; Morteratsch (N46.43340 E9.93623, alt. c. 2002 m) on sandy soil in scrub under Salix sp., 13 Aug. 2005, H. Beker, M.L. Beker HJB11133.
Notes — Given the shape of its cheilocystidia, Hebeloma alpinum clearly belongs to H. subsect. Denudata. Hebeloma alpinum most likely corresponds to ICG4 of Aanen & Kuyper (1999). As discussed in the Discussion in more detail, some members of this species might be intercompatible with H. eburneum (ICG3) or even with H. aanenii (ICG2) (Aanen & Kuyper 1999, Aanen et al. 2000). Hebeloma alpinum certainly appears to be confined to alpine or arctic habitats and can be readily separated from other alpine/arctic species in this section based on the number of lamellae, usually between 40 and 60 on average, and the size of the spores, on average ≥ 11 μm long and > 6 μm wide. It also has quite a robust stature. Microscopically it is closest to H. minus but it can normally be separated on macroscopic characters, since H. minus is smaller with darker coloured pileus and with fewer lamellae than H. alpinum. Hebeloma alpinum is the most common Hebeloma species we have collected in arctic/alpine areas and can usually be determined, with reasonably high confidence, in the field.
Among the loci applied in this study, not a single one can distinguish H. alpinum on its own. Even when combining a minimum of three out of five loci (Fig. 3), H. alpinum is not monophyletic, though, apart form HJB11051 and HJB11545 (discussed in detail above and under H. geminatum), H. alpinum does not form mixed clades with other taxa. Again, the combination of V6 and V9 is probably the most reliable combination of fewer loci for identifying H. alpinum. Hebeloma alpinum V6 sequences cluster with H. geminatum and V9 sequences with H. eburneum, whereas H. geminatum V9 sequences are much longer and the great majority forms a monophylum, except for the two deviant collections, and H. eburneum V6 sequences cluster with H. aanenii.
We have examined material of Naucoria bellotiana (K K(M)165365). This collection from Bellot Island in Canada was collected by Capt. Feilden on 14 August 1876 and described by M.J. Berkeley as Agaricus (Naucoria) bellotianus in the Journal of the Linnean Society, vol. 17, 1878, p. 14. This species certainly belongs to the genus Hebeloma and we make the new combination here:
Hebeloma bellotianum (Berk.) Beker & U. Eberh. comb. nov. — MycoBank MB809913
Hebeloma bellotianum has cheilocystidia that clearly place it in H. sect. Denudata subsect. Denudata. Given the habitat at N81.68 it falls into the arctic/alpine group from this subsection. The number of lamellae (estimated from the exsiccata) and spore size would mean that it would key out, among known species from this subsection, as H. alpinum to which it is certainly similar and were it not for the very large spores we would be confident this was the same species. However the spores of H. bellotiana measured, on average, 14.7 × 7.1 μm and the largest average spore size we have measured for H. alpinum (across 71 collections) is 13.7 μm long (and 7.7 μm wide). We have not been able to obtain any molecular data from this collection and cannot rule out that this may be a species from this subsection that we have not yet encountered. Thus at this point we hesitate to synonymise these species and await further evidence one way or the other.
Hebeloma aurantioumbrinum Beker, Vesterh. & U. Eberh., sp. nov. — MycoBank MB809906; Fig. 5b, 10, 11
Fig. 10.
Hebeloma aurantioumbrinum (BR-MYCO 173985-64, holotype). a, b. Spores and spore ornamentation ×1 600 in Melzer’s reagent; c. cheilocystidia ×1 000 in 5 % KOH; d. cheilocystidia ×500 in 5 % KOH; e. basidium ×1 000 in 5 % KOH; f. epicutis hyphae ×1 000 in 5 % KOH; g. caulocystidia ×1 000 in 5 % KOH; h. caulocystidia ×500 in 5 % KOH. — Scale bars: 10 μm.
Fig. 11.
Hebeloma aurantioumbrinum (BR-MYCO 173985-64, holotype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
Etymology. From aurantio - orange and umbrinum - umber.
Type. SVALBARD, Knudsenheia (N78.9373333 E11.8425333, alt. c. 9 m) on grazed scrub with Salix polaris, 19 Aug. 2007, M.L. Beker, H. Beker, holotype BR BR-MYCO 173985-64; isotypes C C-F-90148, HJB12058.
Diagnosis — Hebeloma aurantioumbrinum possesses the cheilocystidia typical of H. subsect. Denudata. It is a species typically occurring in alpine or arctic habitats where it can be recognized by the combination of an average spore width of < 7 μm and an average cheilocystidium apex width of < 8.5 μm. Outside these habitats it can be differentiated from other small species of the subsection (H. luteicystidiatum, H. helodes and H. pusillum) by its uniformly brownish orange pileus and by its cheilocystidium apex without abnormal wall thickening.
Basidiomes usually in scattered groups. Pileus up to 21 mm diam, umbonate, slightly tomentose at high magnification; surface slightly viscid, tacky when moist rarely hygrophanous; cuticle colour from yellow brown to cinnamon to umber but with some orange, sometimes with a thin paler pinkish buff to clay buff margin; pileus margin usually straight, sometimes slightly scalloped. Lamellae emarginate, quite widely spaced (L = 26–39) with a maximum depth of 2.5 mm; colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, paler than lamella surface; droplets on the lamella edge are usually present and visible to the naked eye, however in dry conditions they may not be seen; lamellules sparse. Stipe central, cylindrical occasionally clavate, stuffed, (14–)15–28 × 2.5–3.1(–3.5) mm and up to 6 mm at the base; white or alutaceous, with no visible discolouring when handled; surface dry, pruinose particularly towards the apex. Cortina not observed. Flesh rather thin, cream or pale brown. Smell raphanoid, sometimes weakly. Taste not recorded. Spore deposit clay-buff.
Spores amygdaloid, with small apiculus and rounded at the end opposite the apiculus, with a distinct thinning of the spore wall but rarely with any papilla, guttulate with one or more oily drops, almost smooth to very weakly ornamented but occasional spores more distinctly ornamented, sometimes with some sign of loosening perispore in a few spores and weakly dextrinoid but sometimes distinctly so (O1,O2(O3); P0,P1; D1,D2); spore colour under the microscope pale brown to yellow brown to brown; spore size based on n = 96 spores of the holotype, 5–95 % percentile range 10.7–12.3 × 6.0–7.1 μm, with median 11.5 × 6.6 μm and av 11.5 × 6.5 μm with SD length 0.47 μm and width 0.33 μm, Q value 5–95 % percentile range 1.65–1.89, with median 1.76 and av 1.76 with SD 0.07; spore size based on 12 collections medians 10.1–11.7 × 6.2–6.8 μm and av 10.2–11.8 × 6.2–6.8 μm with SD length 0.47–0.97 μm and width 0.25–0.34 μm, Qav 1.60–1.85. Basidia cylindrical to clavate and 4-spored, 25–43 × 5.7–11.0 μm, with av 28–38 × 7.1–9.4 μm. Pleurocystidia not found. Cheilocystidia clavate, clavate-stipitate and sometimes spathulate-stipitate, occasionally slightly swollen towards the base (clavate-lageniform) and occasionally with septa, some median thickening or bent; width of apex holotype 5–95 % percentile range 6.1–10.1 μm, with median 8.1 μm and av 8.1 μm with SD 1.19 μm; across 12 collections median 7.1–8.4 μm and av 7.2–8.4 μm; with n ≥ 20 selected cheilocystidia of 12 collections the 5–95 % percentile ranges are 39–71 × 5.3–10.2 × 2.8–5.7 × 2.8–7.1 μm while the averages are 45–61 × 7.2–8.4 × 4.0–4.8 × 4.3–5.4 and 52 × 8.1 × 4.3 × 4.3 μm av for the holotype. The av cheilocystidia ratios for the 12 collections were: A/M = 1.61–1.95; A/B = 1.62–1.98; B/M = 0.97–1.18. Caulocystidia resemble cheilocystidia, up to 75 μm long and 10 μm wide at the apex. Pileipellis is an ixocutis with a medium thick epicutis up to a maximum of 100 μm, embedded hyphae up to 6 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis orange brown and made up of cylindrical to isodiametric elements. Trama below the subcutis contains ellipsoid elements up to 6 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — As well as the 12 collections used in the above description, we also have additional non-European collections from Greenland, the Russian Federation and further collections from the United States. To date all our collections of H. aurantioumbrinum have been collected from alpine or arctic habitats with one collection from a boreal habitat. In every instance, apart from the boreal collection, Salix has been recorded as present. The boreal collection was recorded with Betula sp. and Picea sp. As Salix spp. are easily overlooked, we suspect that Salix was also present although we cannot confirm it. Furthermore, many of our collections have been recorded in wet or damp places. So, while this species has a clear preference for alpine or arctic environments it can also be present in boreal environments if the conditions are appropriate.
Additional specimens examined. ICELAND, Egilsstadir (c. N65.17 W14.23, alt. c. 250 m) arctic, scattered near lakeside on wet clayey soil with Salix sp., 23 July 1987, H. Hallgrimsson HH11047, duplicate HJB11845. – NORWAY, Bollesteinseggi, W of Sogndal Valley, Sogndal (c. N61.22 E6.97, alt. c. 400 m) scattered in an alpine habitat with Salix herbacea, 10 Sept. 2000, E. Bendiksen JV00-291, duplicate HJB10906; Nesseby (c. N70.17 E28.53, alt. c. 120 m) arctic, scattered on boggy mossy ground with Salix sp., 25 Aug. 1998, R. Pöder, B. Pernfuss IB 19980462, database record HJB11835. – SVALBARD, Ny Ålesund (N78.9164333 E11.9717000, alt. c. 12 m) on soil in grazed valley with Salix polaris, 16 Aug. 2007, M.L. Beker, H. Beker HJB12012; Longyearbyen (N78.2113500 E15.6216000, alt. c. 56 m) solitary on urban grazed scrub with Salix polaris, 9 Aug. 2007, M.L. Beker, H. Beker HJB11934. – SWEDEN, Medelpad, Sattna parish, Norrbacken (c. N59.73 E18.01, alt. c. 20 m) boreal, scattered in mixed woodland on wet mossy soil with Betula sp., Picea sp. and Salix sp., 22 Aug. 1995, J. Vesterholt, J.H. Petersen JV95-314, duplicate HJB12310. – USA, Wyoming, Beartooth Plateau, Wyoming Creek (N44.98715 W109.41104, alt. c. 3176 m) alpine, acid soil on scrubland with Salix arctica and S. glauca, 6 Aug. 2008, C. Cripps, H. Beker HJB12445; Wyoming, Beartooth Plateau, Wyoming Creek (N44.9870700 W109.4111100, alt. c. 3177 m) on soil in scrubland under Salix arctica, S. glauca, 6 Aug. 2008, H. Beker HJB12448; Wyoming, Beartooth Plateau, Wyoming Creek (N44.9870700 W109.4111100, alt. c. 3177 m) on mossy, wet soil in scrubland under Salix arctica, S. glauca, 6 Aug. 2008, H. Beker HJB12451; Wyoming, Beartooth Plateau, Wyoming Creek (N44.9871500 W109.4110400, alt. c. 3176 m) on litter and soil in scrubland under Salix arctica, S. glauca, 6 Aug. 2008, C. Cripps HJB12446; Wyoming, Beartooth Plateau, Upper Wyoming Creek (N44.9871500 W109.4110400, alt. c. 3176 m) on mossy, wet soil in scrubland under Salix arctica, 8 Aug. 2008, J. Antibus HJB12456.
Notes — Given the shape of its cheilocystidia, H. aurantioumbrinum clearly belongs to H. subsect. Denudata. It appears to be confined to boreal, alpine or arctic habitats. The species has not been tested for intercompatibility by Aanen & Kuyper (1999). In the past it has probably been overlooked or confused with H. minus or H. subconcolor. It can be distinguished from H. minus through the average width of the cheilocystidia apex which ≤ 8.5 μm for H. aurantioumbrinum while the average cheilocystidium apex for H. minus is always > 8.5 μm. With regard to H. subconcolor, this species is from H. sect. Velutipes and as such has cheilocystidia of the velutipes-type hence more gently clavate and with a lower average value for ratio A/M, rarely > 1.5. In determining H. aurantioumbrinum the combination of characters is important. As a small Hebeloma sp. in an alpine or arctic habitat with the distinctive subsect. Denudata shaped cheilocystidia there are few possibilities and the average spore width (< 7 μm) and the average cheilocystidium apex (< 8.5 μm) means it can only be H. aurantioumbrinum. In the case when it might be collected in a boreal rather than alpine or arctic habitat (we have only seen one such collection), then the small size of mushroom, the apex of the cheilocystidia that does not show any abnormal thickening and the more or less unicoloured brownish orange pileus separates this species from the other small species of H. subsect. Denudata, namely: H. luteicystidiatum, H. helodes and H. pusillum.
Molecularly, H. aurantioumbrinum is sister species to H. helodes from which it can only be separated based on RPB2 and MCM7. With either of these, H. aurantioumbrinum forms a well-supported species clade.
Hebeloma eburneum Malençon, Champignons Supérieurs du Maroc 1: 445. 1970. — MycoBank MB314954; Fig. 12, 13
Fig. 12.
Hebeloma eburneum (MPU GM1122, holotype). a, b. Spores and spore ornamentation ×1 600 in Melzer’s reagent; c, d. spores and spore ornamentation ×1 600 in 5 % KOH. — H. eburneum (HJB12772). e, f. Cheilocystidia ×1 000 in 5 % KOH; g. basidia at gill edge ×500 in 5 % KOH; h. caulocystidia ×500 in 5 % KOH. — Scale bars: 10 μm.
Fig. 13.
Hebeloma eburneum (MPU GM1122, holotype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
= Hebeloma perpallidum M.M. Moser, Z. Pilzk. 36, 1-2: 72. 1970.
= Hebeloma ochroalbidum Bohus, Ann. Hist.-Nat. Mus. Natl. Hung. 64: 71. 1972.
= Hebeloma albocolossum M.M. Moser, Sydowia 38: 174. 1986 [1985].
= Hebeloma crustuliniforme var. tiliae Bres., Z. Mykol. 53, 2: 294. 1987.
Type. MOROCCO, Azrou (c. N33.417 W5.217, alt. c. 1500 m) with Cedrus libanotica ssp. atlantica, 8 Nov. 1941, G. Malençon, MPU GM1122, database record HJB1000095.
Basidiomes usually in scattered groups, sometimes solitary, sometimes caespitose. Pileus (8–)20–133 mm diam, convex often umbonate, surface often viscid, tacky when moist but never hygrophanous; cuticle colour always pale from pale cream to buff or buff yellow occasionally with the centre slightly yellow brown or light ochre or Isabella but then becoming paler towards the margin which is always pale cream or slightly yellowish; pileus margin often involute particularly in young basidiomes but then straightening in older basidiomes, rarely serrate. Lamellae emarginate or adnate, usually crowded with L = 70–110 but we do have some collections with L = 40–70, so unusually within the genus this character appears quite variable for this species; maximum depth up to 9 mm; colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, usually significantly paler than lamella surface; droplets normally very visible on the lamella edge; lamellules frequent. Stipe central, sometimes cylindrical but often clavate and rarely tapering, becoming hollow with age and very rarely with a superior wick, 20–106 × (3–)6–29(–36) mm, sometimes with slight yellowish discolouration towards the base of the stipe; surface dry, pruinose to distinctly floccose, especially towards the apex. Cortina not observed. Flesh thick, white to pale buff. Smell raphanoid, sometimes weak, sometimes absent and occasionally a hint of cocoa. Taste mild to weakly bitter with some raphanoid components. Spore deposit brownish olive to Isabella. Exsiccata with no special features but sometimes very pale.
Spores amygdaloid, sometimes weakly limoniform or fusoid, with small apiculus and rounded at the end opposite the apiculus, with some thinning of the spore wall, and rarely with a distinct papilla, sometimes guttulate with one or more oily drops, sometimes weakly ornamented but usually more distinctly ornamented, sometimes with slightly loosening perispore in a few to many spores and weakly but usually distinctly dextrinoid (O2,O3; (P0)P1(P2); D1,D2); spore colour under the microscope brown, sometimes with a yellow tinge; spore size based on n = 53 spores of the holotype, 5–95 % percentile range 10.4–12.8 × 6.0–7.0 μm, with median 11.7 × 6.5 μm and av 11.6 × 6.5 μm with SD length 0.69 μm and width 0.33 μm, Q value 5–95 % percentile range 1.65–1.98, with median 1.78 and av 1.80 with SD 0.11; spore size based on 38 collections medians (10.3–)10.9–13.7 × (5.5–)6.1–7.1 μm and av (10.3–)10.9–13.7 × (5.5–)6.1–7.0 μm with SD length 0.49–0.94 μm and width 0.23–0.49 μm, Qav (1.60–)1.69–2.06. (It should be noted that among the collections examined was one collection with unusually small spores.) Basidia cylindrical to clavate and 4-spored, 23–42 × 6.5–11.7 μm, with av 24–37 × 7.1–10.4 μm and basidium Qav in the range 2.9–4.4. Pleurocystidia not found. Cheilocystidia capitate-stipitate, clavate-stipitate or occasionally spathulate-stipitate, sometimes swollen towards the base (capitate-lageniform or clavate-lageniform), often conglutinate, sometimes with thickening of the apex or thickening of the median, sometimes septate, occasionally sinuate, bifurcate or with yellowish contents; width of apex holotype 8–10 μm (according to Malençon as we were unable to find cheilocystidia when we examined the exsiccata); across 38 collections median 7.8–10.3 μm and av 8.0–10.4 μm; with n atleast 20 selected cheilocystidia of 38 collections the 5–95 % percentile ranges are 35–93 × 6.1–13.2 × 2.8–6.8 × 2.6–7.7 μm while the averages are 45–71 × 8.0–10.4 × 3.6–4.9 × 3.7–5.1. The av cheilocystidia ratios for the 38 collections were: A/M = 1.78–2.71; A/B = 1.80–2.72; B/M = 0.90–1.19. Caulocystidia resemble cheilocystidia, up to 90 × 11 μm wide at the apex. Pileipellis is an ixocutis with an epicutis medium thick, 80–120 μm, embedded hyphae up to 6 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis cream to yellow and made up of cylindrical to isodiametric elements. Trama below the subcutis contains angular, cylindrical and sausage-shaped elements up to 18 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — Hebeloma eburneum has been recorded with a wide variety of trees, primarily broadleaf but occasionally also with conifers. In Malençon’s original description he claims it is uniquely associated with Cedrus but we have numerous collections where no Cedrus was present. Trees recorded as associates include: Betula spp., Carpinus betulus, Cedrus spp., Fagus sp., Larix sp., Picea sp., Pinus sp., Populus sp., Quercus sp., Salix sp., Tilia sp. It has been recorded in various types of woodland, both broadleaf and conifer, parklands, churchyards, urban areas, dunes, Salix thickets, often close to paths. Habitats include grassy places as well as exposed and overgrazed alvar. We have no records of this species from arctic or alpine habitats. The species occurs in calcareous, chalky, sandy and clayey, both wet and dry soil conditions.
Additional specimens examined. AUSTRIA, Tirol, Silz, Forstgarten (c. N48.38 WE16.72, alt. c. 150 m) in plantation with Picea sp. and Pinus sylvestris, 18 Oct. 1962, F. Gobl IB 19620069, database record HJB1000071; this is the holotype of H. perpallidum. – BELGIUM, Brussels, Foret de Soignes (N50.7950333 E4.4332833, alt. c. 114 m) in broadleaf woodland on calcareous soil with Fagus sylvatica, 8 Sept. 2006, H. Beker HJB11687; Foret de Soignes (N50.79578333 E4.4297333, alt. c. 100 m) in broadleaf woodland on calcareous soil with Fagus sylvatica, 8 Sept. 2006, H. Beker HJB11690; Zaventem (c. N50.89 E4.46, alt c. 40 m) in grassland under Betula sp., 8 Oct. 2005, D. Deschutyer HJB11235; prov. Luxembourg, Reine (N50.30185 E5.4318333, alt. c. 180 m) in mixed woodland with Picea sp., 8 Oct. 2004, M. Lenne HJB10290. – CZECH REPUBLIC, LPA Moravian Karst, NNR Vyvery Punkvy (N49.37245 E16.73003, alt. c. 490 m) in mixed woodland on calcareous grassy soil under Populus sp., 10 Oct. 2008, S. Kelly HJB12719; LPA Moravian Karst, NNR Vyvery Punkvy (N49.3724500 E16.7300300, alt. c. 487 m) on soil in mixed woodland scrub under Populus sp., 10 Oct. 2008, S. Kelly HJB12720. – DENMARK, Brabrand W of Århus, Egebjergvej (c. N56.15 E10.10, alt. c. 30 m) on grassy soil with Pinus sp., 6 Oct. 1983, J. Vesterholt C He83-207, duplicate HJB10940; Vejle, Nordre Kirkegård (c. N55.719 E9.555, alt. c. 50 m) with Tilia sp., 12 Sept. 2004, J. Vesterholt C JV04-245; duplicate HJB10708; Vejle, Bredballe N, Solgave Alle (N55.72682 E9.5850860, alt. c. 70 m) on dry clayey soil in urban broadleaf scrub under Salix sp., 11 Oct. 1991, J. Vesterholt C JV91-586, duplicate HJB12359. – ENGLAND, South Lancashire, Ainsdale (c. N53.59273 W3.054548, alt. c. 0 m) on sandy soil in dune with Salix sp. and Populus sp., 22 Sept. 2002, M. Rotheroe HJB9267. – ESTONIA, Saaremaa, Salme commune, Kaugatoma-Löo (N58.09321 E22.17693, alt. c. 5 m) on alvar soil in maritime scrub under Betula sp., Pinus sylvestris, 18 Sept. 2008, J. Vesterholt JV08-251, HJB12327; Hiiumaa, Pühalepa commune, Sarve peninsula, Soonlepa (N58.85049 E23.03069, alt. c. 10 m) in mixed woodland, on grassy calcareous soil with Betula pubescens, Picea abies and Pinus sylvestris, 25 Sept. 2008, J. Vesterholt C JV08-408; duplicate HJB12338; Hiiumaa, Körgessare Commune, Reigi cemetery (N58.98308 E22.51068, alt. c. 10 m) in churchyard lawn on dry calcareous soil under Betula pubescens and Larix sp., 25 Sept. 2008, J. Vesterholt C JV08-405, duplicate HJB12343. – FINLAND, Turku town, Mäntymäki, near the cross of Vähä-Heikkiläntie and Luolavuorentie (c. N60.64505 E22.27442, alt. c. 40 m) on lawn under Tilia sp., 22 Sept. 2005, J. Vauras C TURA JV23655, duplicate HJB12399. – FRANCE, Vienne, Saint-Bermier (N46.4542667 W0.0174167, alt. c. 117 m) in parkland on grassy soil under Populus sp., 4 Nov. 2004, H. Beker HJB10525; Provence, Le Verdiere (c. N43.94 E4.877, alt. c. 30 m) with Pinus halepensis and Quercus ilex, 9 Nov. 1999, P.-A. Moreau C JV99-720, duplicate HJB10899. – GERMANY, Herrnhut, Herrschaftsgarten (c. N51.0110430 E14.7508300, alt. c. 330 m) under Carpinus betulus, 29 Oct. 1999, G. Zschieschang GLM GL40795, database record HJB10979; Lossa (c. N51.220 E11.410, alt. c. 310 m) under Betula sp., Populus sp., Salix sp., 24. Oct. 2004, D. Penke GLM: HBo79138, duplicate HJB12243; Parkplatz, Hilpoltstein, Bayern (c. N49.189 E11.184, alt. c. 380 m) with Tilia sp., 24 Sept. 1995, D. Aanen WBS 9511, database record HJB12501; Regensburg, Universitätsgelände (c. N49.00 E12.09, alt. c. 370 m) with Tilia sp., 4 Oct. 1978, A. Bresinsky M M5340, database record HJB13014. – HUNGARY, Fot Com. Pest (c. N47.50 E19.40, alt. c. 220 m) in broadleaf woodland on sandy soil under Populus sp., 20 Oct. 1971, I. Schumeth BP 48.427, database record HJB1000052, this is the holotype of H. ochroalbidum. – MACEDONIA, Ezerani near Asamati Village (N40.99356 E21.03927, alt. c. 850 m) in Salix thicket on calcareous soil with Salix fragilis and S. amplexicaulis, 10 Nov. 2008, H. Beker HJB12769, duplicate C JV08-451; Ezerani near Asamati village (N40.99359 E21.03931, alt. c. 855 m) in Salix thicket under Salix fragilis, Salix sp., 10 Nov. 2008, H. Beker HJB12771; Ezerani near Asamati village (N40.99320 E21.03889, alt. c. 854 m) in Salix thicket under Salix fragilis, Salix sp., 10 Nov. 2008, J. Vesterholt JV08-452, HJB12772. – MOROCCO, Azrou Clairicie de Boudmeme (c. N33.4167 W5.2157, alt. c. 1740 m) with Cedrus sp., 1 Nov. 1943, G. Malençon MPU GM1415, database record HJB12229. – NETHERLANDS, Limburg, Elsloo (c. N50.57 E5.46001, alt. c. 65 m) with Populus sp., 25 Oct. 1996, D. Aanen WBS 9699, database record HJB12537; Zuid-Holland, Hoornaar, Grootewaard (c. N51.8767 E4.9469, alt. c. 0 m) with Populus sp., 20 Oct. 1996, D. Aanen WBS 9693, database record HJB12536); Zuid-Holland, Hoornaar, Grootewaard (c. N51.8767 E4.9469, alt. c. 0 m) with Populus sp., 15 Oct. 1996, D. Aanen WBS 9680, database record HJB12480; Zuid-Holland, Hoornaar, Grootewaard (c. N51.8767 E4.9469, alt. c. 0 m) with Populus sp., 15 Oct. 1996, D. Aanen WBS 9679, database record HJB12804; Utrecht, Lunetten (c. N52.03453 E5.07562, alt. c. 4 m) with Salix alba, 13 Oct. 1996, D. Aanen WBS 9686, database record HJB12996. – POLAND, Mt Kamiensk (the outer damping ground of the Belchatow Lignite Mine), forest distr. 300b (near the cross, plot Z-5d, 12 year old plantation) (N51.21337 E19.44114, alt. c. 372 m) conifer woodland plantation with Pinus sylvestris and Quercus sp., 23 Sept. 2008, I. Kałucka, H. Beker HJB12667; Mt Kamiensk (the outer damping ground of the Belchatow Lignite Mine), forest distr. 300b (near the cross, plot Z-5d, 12 year old plantation) (N51.21322 E19.44148, alt. c. 370 m) conifer woodland plantation with Pinus sylvestris, Betula sp. and Alnus sp., 23 Sept. 2008, I. Kałucka, H. Beker HJB12670; Jelonka reserve (undisturbed), plot 16 (phase 8) (c. N52.596 E23.363, alt. c. 180 m) in naturally regenerated conifer woodland on abandoned fields with Pinus sylvestris, Populus tremula and Salix sp., 9 Oct. 1996, I. Kałucka LOD 19481, database record HJB13070. – SWEDEN, Hammarskog, Dalkarlskärret, Uppsala (c. N59.77 E17.55, alt. c. 20 m) in mixed woodland with Populus sp., 29 Sept. 2003, A. Taylor UPS AT2003093, duplicate HJB10667; north shore of Tornesträsk, Norbotten (c. N66.943 E19.81, alt. c. 420 m) with Betula tortuosa, 25 Aug. 1981, M. Moser IB 19810313, database record HJB1000069, this is the holotype of H. albocolossum. – SWITZERLAND, Bern, Gampelen (c. N47.01 E7.05, alt. c. 520 m) in mixed woodland on wet soil with Populus sp., 19 Sept. 1993, J. Vesterholt C JV93-943, duplicate HJB10840.
Notes — Given the shape of its cheilocystidia, H. eburneum clearly belongs to H. subsect. Denudata. The species most likely corresponds to ICG3 of Aanen & Kuyper (1999). It appears to have a wide range both geographically and ecologically. There is no morphological or molecular evidence to separate this species from H. albocolossum, H. ochroalbidum and H. perpallidum, which were all described as large and fleshy with a very pale coloured cap. Moser saw the primary differences as being the presence or absence of tears on the lamellae and the discolouration of the stipe. He regarded H. eburneum as a Mediterranean species and the others as more northern. He also believed spore size and the presence of a papilla on the spores, leading to a more limoniform shape, as significant (Moser 1970, 1985). The presence or absence of tears can be affected by the weather and while it is a good character it is not a dependable character. The discolouration of the stipe is also an important character but again can be affected by local ecology; for this species the stipe does not usually discolour very much with age, however, when conditions are very damp the stipe can exhibit some discolouring which starts from the base of the stipe. This species is morphologically close to H. aanenii and to H. geminatum but usually has rather larger spores; we have had one collection of H. eburneum that has smaller spores than usual and if the spores are both short and narrow it may become impossible to separate these species purely on the basis of the spore size. Hebeloma eburneum is also morphologically close to H. crustuliniforme but can be distinguished through its cheilocystidia, which have on average a larger apex. Hebeloma crustuliniforme also tends to have a rather more coloured cap.
If species identification was attempted with a single locus only, then RPB2 was probably the most reliable among the loci tested. The majority of H. eburneum ITS sequences form a H. eburneum clade (Fig. 1a). Most of the H. eburneum sequences placed outside this clade are phased copies from collections with length differences within their ITS. These sequences are in the unresolved part of the tree, together with H. alpinum and H. geminatum sequences. Typically, only one of the two phased alleles of a collection falls outside the H. eburneum clade. The only exception to this is the type of H. albocolossum where both alleles, if phased (results not shown as there are only ambiguities, no length difference in the direct ITS reads), are in the unresolved part of the tree. We did not obtain many RPB2 sequences, but the results obtained so far argue against hybridization and suggest that the presence of unspecific ITS alleles in H. eburneum is more likely a consequence of incomplete lineage sorting than of frequent hybridization events.
As in H. alpinum, the combination of V6 and V9 is another option for separating H. eburneum from other members of the H. crustuliniforme complex, because H. eburneum V6 sequences form mixed clades with H. aanenii and V9 sequences with H. alpinum, and the H. alpinum V6 sequences or the H. aanenii V9 sequences, respectively, are clearly different from H. eburneum based on presence/absence patterns of indels.
In deciding on the priority of the name H. eburneum a number of factors have been taken into account:
Hebeloma eburneum and H. perpallidum both have publication dates of 1970 (Malençon & Bertault 1970, Moser 1970). Hebeloma ochroalbidum has an effective publication date of 1972 (Bohus 1972) and H. albocolossum has an effective publication date of 1986 (Moser 1985).
Hebeloma perpallidum was published in Z. Pilzk. vol. 36, Parts 1 & 2. We have been informed by the publishers that this was published in December 1970.
Hebeloma eburneum was published in the Flore des Champignons Superieurs du Maroc Vol. 1 which is Volume 32 of Trave. Inst. Sci. Cherifien de la Fac. Sci. Rabat. We have not been able to establish a precise date of publication for this volume but:
Hebeloma eburneum was listed in Index of Fungi 4, 3: 65 (early 1972); but perpallidum was not listed until a year later, in Index of Fungi 4, 5: 133 (early 1973).
The book by Malençon & Bertault was reviewed in Bull. Soc. Mycol. France 86, 3: 793 on 7 May 1971 and most other publications reviewed in that issue of the journal have a publication date of 1969, or even 1968. This suggests that the book was published relatively early in 1970 and almost certainly not as late as December 1970.
The holotype of H. crustuliniforme var. tiliae is a mixed collection. The part of the collection that corresponds best with the species description is designated here as lectotype of the species (M M-0151680, database record HJB13014, MycoBank MBT198154).
Hebeloma geminatum Beker, Vesterh. & U. Eberh., sp. nov. — MycoBank MB809907; Fig. 5c, 14, 15
Fig. 14.
Hebeloma geminatum (C-F-90152 (JV96-341, holotype)). a, b. Spores and spore ornamentation ×1 600 in Melzer’s reagent; c, d. spores and spore ornamentation ×1 600 in 5 % KOH; e. cheilocystidia ×500 in 5 % KOH; f. cheilocystidia ×1 000 in 5 % KOH; g. basidia ×1 000 in 5 % KOH; h. caulocystidia ×500 in 5 % KOH; i. caulocystidia ×1 000 in 5 % KOH. — Scale bars: 10 μm.
Fig. 15.
Hebeloma geminatum (C-F-90152 (JV96-341, holotype; database record HJB HJB10833)).
Etymology. From the base geminus meaning a double or twin and to emphasise that this is one of two taxa that can be differentiated molecularly and biologically but for which we have found no unambiguous morphological character to separate them.
Type. DENMARK, Nystrup Klitplantage, S of Kridtstien (N57.00 E8.52, alt. c. 42 m) with Abies sp., 10 Oct. 1996, J. Vesterholt holotype C JV96-341; isotype BR BR-MYCO 173983-62, database record HJB10833.
Diagnosis — Hebeloma geminatum with primarily capitate-, clavate- or spathulate-stipitate cheilocystidia belongs to H. subsect. Denudata. It can be distinguished from other taxa of the section by its average number of lamellae, which is ≥ 60, its spores, which are on average between 6 and 6.4 μm wide and < 10.75 μm long, and the cheilocystidia apex, which is always on average > 8 μm and often > 9 μm. The species is morphologically most similar to H. aanenii from which it can be distinguished by comparison of the sequence of the internal transcribed spacer region of the nuclear ribosomal RNA genes.
Basidiomes usually in scattered groups, sometimes solitary, sometimes caespitose. Pileus up to 120 mm diam, convex, sometimes umbonate or applanate; surface slightly viscid, tacky when moist never hygrophanous, sometimes rugulose; cuticle colour usually from white to cream to buff, sometimes yellowish, becoming paler towards the margin which is always very pale; pileus margin usually straight, but occasionally involute or crenulate, upturned with age. Lamellae emarginate, adnexed or adnate, crowded (L = 65–100) with a maximum depth of 4–8 mm; colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, paler than lamella surface; droplets on the lamella edge are usually present and visible to the naked eye; lamellules frequent. Stipe central, cylindrical, often clavate, (15–)27–110 × 6.5–13 mm and up to 16 mm at the base; white or alutaceous, sometimes but not always discolouring from the base when handled or with age; surface dry, strongly floccose particularly towards the apex; interior stuffed when young but sometimes becoming hollow with age. Cortina not observed. Flesh rather thick, cream or pale brown. Smell raphanoid. Taste raphanoid, usually mild. Spore deposit greyish brown to brownish olive.
Spores amygdaloid, with small apiculus and rounded at the end opposite the apiculus, with a distinct thinning of the spore wall but rarely with any papilla, guttulate with one or more oily drops, weakly ornamented to distinctly ornamented, sometimes with some sign of loosening perispore in a few spores and weakly dextrinoid (O2,O3; (P0)P1(P2); D0,D1); spore colour under the microscope yellow- or grey-brown to brown; spore size based on n = 63 spores of the holotype, 5–95 % percentile range 9.2–11.7 × 5.4–6.9 μm, with median 10.3 × 6.0 μm and av 10.4 × 6.0 μm with SD length 0.85 μm and width 0.51 μm, Q value 5–95 % percentile range 1.58–1.89, with median 1.74 and av 1.74 with SD 0.1; spore size based on 25 collections medians 9.8–10.8(–11.6) × 5.4–6.4 μm and av 9.8–10.8(–11.6) × 5.4–6.3 μm with SD length 0.38–1.02 μm and width 0.19–0.51 μm, Qav 1.65–1.93. Basidia cylindrical to clavate and 4-spored, 22–38 × 6.6–9.9 μm, with av 26–35 × 7.1–9.0 μm. Pleurocystidia not found. Cheilocystidia capitate-, clavate- and sometimes spathulate-stipitate, occasionally slightly swollen towards the base (clavate-lageniform) and occasionally with some median thickening, sinuate, septate or bifurcate; width of apex holotype 5–95 % percentile range 6.8–12.2 μm, with median 9.4 μm and av 9.4 μm with SD 1.74 μm; across 25 collections median 8.0–10.4 μm and av 8.0–10.4 μm (of the 25 collections 10 have the cheilocystidium apex av ≥ 9 μm); with n ≥ 20 selected cheilocystidia of 25 collections the 5–95 % percentile ranges are 36–91 × 6.2–13.2 × 2.8–6.2 × 2.4–7.2 μm while the averages are 50–72 × 8.0–10.4 × 4.0–4.7 × 3.7–5.0 and 55 × 9.4 × 4.2 × 5.0 μm av for the holotype. The av cheilocystidia ratios for the 19 collections were: A/M = 1.76–2.57; A/B = 1.68–2.85; B/M = 0.81–1.19. Caulocystidia resemble cheilocystidia, up to 70 μm long and 12 μm wide at the apex. Pileipellis is an ixocutis with a medium thick epicutis 110–200 μm, embedded hyphae up to 5–8 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis yellowish and made up of cylindrical to isodiametric elements. Trama below the subcutis contains cylindrical, ellipsoid, spherical and thick sausage shaped elements up to 16 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — Hebeloma geminatum has been recorded with a variety of trees including: Abies, Betula, Corylus, Fagus, Picea, Pinus, Populus, Quercus, Salix, Tilia, on various acid and neutral soils, often sandy or mossy and in rotten litter, in a range of woodlands and plantations, often on roadsides or pathsides, as well as in gardens and parklands and also on slagheaps.
Additional specimens examined. BELGIUM, prov. Brussels, Foret de Soignes (N50.790 E4.430, alt. c. 114 m) on rotten litter in broadleaf woodland under Fagus sp., 16 Sept. 2003, H. Beker HJB8633; prov. Antwerp, Geel, Dekshoevevijver IFBL: C6.21.34 (c. N51.1667 E5.000, alt. c. 20 m) under Populus sp., 19 Oct. 2003, J. Volders VJ03073, duplicate HJB10969; prov. Luxembourg, Ste-Cecile (N49.7632167 E5.2690500, alt. c. 257 m) on rotten litter in broadleaf woodland under Fagus sp., 17 Sept. 2004, M. Lenne HJB10603; prov. Hainaut, Maubray (N50.5302833 E3.4948667, alt. c. 52 m) on rotten litter in broadleaf woodland on slagheap under Betula sp., Populus sp., Salix sp., 10 Nov. 2006, P.-A. Moreau HJB11736. – DENMARK, region NEZ, Tisvilde Hegn, East end (c. N56.020 E12.030, alt. c. 15 m) under Betula sp., Picea sp., 24 Sept. 2003, T. Læssøe TL-11137, HJB10834; region EJ, Als Odde, Sommerland (N56.71199 E10.32148, alt. c. 10 m) on sandy mossy soil in roadside woodland under Betula sp., Larix sp., Picea sp., Pinus sp., Populus tremula, Quercus sp., 30 Aug. 2008, J. Vesterholt JV08-101, HJB12319. – ENGLAND, East Kent, Bedgebury Pinetum (c. N51.280 E0.540, alt. c. 55 m) on soil, 17 Oct. 1971, D.A. Reid K K(M)106232, database record HJB11604; Berkshire, California Park (N51.3813000 W0.8762839, alt. c. 55 m) on wet soil in mixed woodland under Salix sp., 5 Oct. 1997, H. Beker HJB1889; Surrey, Whitley (N51.1570200 W0.6686763, alt. c. 66 m) on rotten litter in mixed woodland under Salix sp., 4 Nov. 2000, H. Beker HJB3808; West Sussex, Graffham (N50.9655900 W0.6798483, alt. c. 52 m) in conifer woodland under Betula sp., 17 Sept. 2002, M. Storey HJB10766; Surrey, La Baraka, Gorse Hill Road (N51.4093800 W0.5650113, alt. c. 70 m) on rotten litter in urban garden under Betula sp., 31 Oct. 2004, H. Beker HJB10384; West Kent, Tudeley Wood (c. N51.1660700 E0.3099706, alt. c. 88 m) on sandy soil in broadleaf woodland under Betula sp., Quercus sp., 22 Oct. 2005, A. Andrews HJB11545; Surrey, La Baraka, Gorse Hill Road (N51.4088400 E0.5650200, alt. c. 51 m) on mossy soil in urban garden under Betula pendula, 28 Oct. 2009, H. Beker HJB13309. – ESTONIA, Hiiumaa district, Puhalepa commune, Sarve (c. N58.180 E22.060, alt. c. 0 m) on alvar soil in woodland path under Betula sp., Corylus avellana, Juniperus sp., Pinus sylvestris, 16 Sept. 2001, J. Vauras 17928F, HJB10961. – FRANCE, Yvelines, St-Leger-en-Yvelines, Foret domaniale de Rambouillet (c. N48.6703 E1.7797, alt. c. 160 m) on sandy soil in conifer woodland plantation under Pinus sp., 3 Nov. 1998, G. Corriol GC98 11 03 02, HJB12934; Nord Pas de Calais, Auberchicourt (N50.3475667 E3.2351667, alt. c. 23 m) on grassy soil in broadleaf woodland slagheap under Betula sp., Salix sp., 13 Nov. 2006, C. Lecuru HJB11801. – NETHERLANDS, Groningen, Eemshaven (c. N53.455 E6.806, alt. c. 0 m) under Salix alba, S. caprea, S. repens, 3 Oct. 1996, D. Aanen WBS 9675, database record HJB12469. – NORWAY, Skibotndalen fieldstation Tromsø (c. N69.260 E20.510, alt. c. 280 m) under Betula pubescens, 6 Aug. 1995, M. Moser (as H. albocolossum) IB 19950057, HJB11829. – POLAND, Mt Kamiensk (N51.2245833 E19.4003611, alt. c. 220 m) on calcareous, clayey, sandy soil in conifer woodland plantation and quarry site under Pinus sylvestris, Populus sp., Salix sp., 2 Nov. 2004, I. Kałucka LOD IK-H0004, HJB13188; Wolka Lekawska, forest distr. 8C k (plot no. P-2), 9 year old pine plantation (75 % pine; 25 % birch) on former arable land (N51.3001500 E19.3972700, alt. c. 200 m) on mossy, sandy soil in mixed woodland plantation under Betula sp., Pinus sylvestris, 22 Sept. 2008, I. Kałucka, H. Beker HJB12655; Bialowieski Park Narodowy (c. N52.72111 E23.90560, alt. c. 150 m) on soil in mixed woodland under Betula sp., 19 Sept. 2008, D. Karasinski, A. Szczepkouski HJB12639. – SWEDEN, Medelpad, Borgsjö (c. N62.560 E15.870, alt. c. 200 m) under Betula sp., 19 Sept. 1995, E. Arnolds WBS 9503, database record HJB12809; Stradssbergen (c. N59.850 E17.610, alt. c. 40 m) on mossy litter in mixed woodland, 24 June 2004, A. Taylor AT2004020, HJB10770; Uppsala, Uppsala Hospital (c. N59.600 E16.540, alt. c. 49 m) on grassy soil in mixed parkland under Picea sp., Pinus sp., Tilia sp., 5 July 2004, A. Taylor AT2004061, HJB10776.
Notes — Given the shape of its cheilocystidia, H. geminatum clearly belongs to H. subsect. Denudata. Hebeloma geminatum most likely corresponds to ICG1 of Aanen & Kuyper (1999) and is a constituent of the ‘Hebeloma crustuliniforme complex’. It is likely that many collections of this species have been recorded under the name H. crustuliniforme and exist worldwide in herbaria under this name. It is morphologically similar to H. aanenii, H. alpinum, H. crustuliniforme and H. eburneum. But its spores, normally < 11 μm long and < 6 μm wide (rarely longer or wider) distinguish it from the first three species. It can be distinguished from other members of this subsection by the number of complete lamellae, which is always ≥ 60.
Until now we have found no consistent morphological character to separate H. aanenii and H. geminatum. However, we can often separate these two taxa through the use of a mixture of characters, although none appears fool proof. The best character appears to be the cheilocystidium apex width; for H. geminatum this is usually larger than that for H. aanenii. From our records, if the average width of the apex of the cheilocystidium is more than 9 μm then the collection is almost certainly H. geminatum. However, the average apex width can be as small as 8 μm and widths in this interval between 8–9 μm can be from either taxon. This is illustrated in the scatter diagram (Fig. 4b).
Of course one can combine characters, so, for instance a collection with large basidiomes exhibiting a thick epicutis in excess of 200 μm and a cheilocystidium apex width of < 8.5 μm will almost certainly be H. aanenii. Similarly, a collection with small basidiomes, a relatively thin epicutis and cheilocystidium apex width of > 8.5 μm will almost certainly be H. geminatum. A synoptic key will be far more powerful as a tool based on morphological characters; but we do not yet feel we have examined sufficient collections to build such a key with confidence.
It is worth noting that we do not have any confirmed records from southern Europe. Other than two Swiss alpine collections we have not seen (and therefore are not cited in the species description, WBS 9618, WBS 9621, corresponding to ICG1, Aanen & Kuyper 1999), our most southerly collection is from France at a latitude of more than N48.67.
Molecularly, H. geminatum and H. alpinum are very close and sequences of both species are paraphyletic in four out of the five loci tested, though, in MCM7 H. geminatum forms clades not including H. alpinum (apart from collection HJB11051 discussed elsewhere). The only locus that normally clearly separates H. geminatum from H. alpinum is V9. Hebeloma alpinum lacks an indel present in H. geminatum and some other members of H. subsect. Denudata. In fact, there is only a single bp difference between some H. aanenii and H. geminatum V9 sequences, but all other loci, including the ITS, can separate those two taxa.
Collection HJB11051 (see also in the Discussion) is genetically closer to H. geminatum in all loci tested than to H. alpinum, but corresponds morphologically with H. alpinum and, being an alpine collection, is also ecologically more typical for the latter species. One could argue that the genetic assignment is more meaningful in terms of ancestry and call the collection H. geminatum. However, as it is morphologically clearly different from typical H. geminatum, we decided not to include it here.
The V9 sequence of H. geminatum HJB11545, in spite of possessing the indel, differs quite strikingly in a number of positions from the ‘normal’ V9 of H. geminatum and in fact from all other V9 sequences obtained from members of its subsection. The placement of HJB11545 in the concatenated analyses has to be interpreted as being outside the H. geminatum clade as opposed to being a member of H. alpinum. Morphologically and ecologically, HJB11545 fits well into H. geminatum, only the spore width with an average of 5.4 μm is an outlier within this taxon.
Hebeloma helodes J. Favre, Beitr. Kryptogamenfl. Schweiz 10, 3: 214. 1948. — MycoBank MB286871; Fig. 16, 17
Fig. 16.
Hebeloma helodes (G 00053920, lectotype). a, b. Spores and spore ornamentation ×1 600 in Melzer’s reagent; c, d. spores and spore ornamentation ×1 600 in 5 % KOH; e. cheilocystidia ×1 000 in 5 % KOH; f. cheilocystidia ×500 in 5 % KOH; g. basidium ×1 000 in 5 % KOH; h. caulocystidium ×500 in 5 % KOH; i. epicutis hyphae ×500 in 5 % KOH. — Scale bars: 10 μm.
Fig. 17.
Hebeloma helodes (G 00053920, lectotype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
Type. SWITZERLAND, Vaud, Jura, Tourbiere du sentier, Vallee de Joux (c. N48.6122 E6.2358, alt. c. 1000 m) in wet soil with Filipendula ulmaria in a bog with Betula sp. and Pinus sp., 30 Aug. 1939, G. Favre 9139, lectotype G 00053920, database record HJB1000054, selected by F. Gröger in Myk. Mittbl. 30, 2: 46, 1987.
Basidiomes usually in scattered groups or sometimes solitary. Pileus 13–38 mm diam, convex, sometimes weakly umbonate, sometimes slightly depressed in the centre especially when older, often viscid, tacky when moist but never hygrophanous; cuticle colour quite pale and cream or white towards the margin with the centre sometimes darker from light ochraceous to dark beige or yellowish brown; pileus margin usually involute although this feature sometimes disappears in older basidiomes. Lamellae emarginate, moderately spaced (L = 37–54) with a maximum depth of 3–5 mm; colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, significantly paler than lamella surface; droplets normally visible on the lamella edge even with the naked eye; lamellules frequent. Stipe central, usually cylindrical but sometimes slightly to distinctly clavate, becoming hollow with age, 15–60 × 3–4.5 mm and up to 7 mm at the base; white or alutaceous, occasionally with some slight brown discolouration at the base of the stipe; surface dry, floccose particularly towards the apex. Cortina not observed. Flesh rather thin, whitish but slightly more coloured in the base of the stipe. The whole basidiome has a very slender appearance with the length of the stipe normally at least twice the width of the pileus. Smell raphanoid, rarely absent. Taste raphanoid, not bitter. Spore deposit Isabella to brownish olive.
Spores amygdaloid, with small apiculus and rounded at the end opposite the apiculus, with a distinct thinning of the spore wall, rarely with any papilla, guttulate with one or more oily drops, from weakly to distinctly ornamented, usually with some sign of loosening perispore in at least a few spores and often in many spores and at most weakly dextrinoid (O2,O3; (P0)P1,P2; DO,D1(D2)); spore colour under the microscope yellow, occasionally with a brown tinge; spore size based on n = 97 spores of the holotype, 5–95 % percentile range 9.0–10.7 × 5.1–6.0 μm, with median 9.8 × 5.6 μm and av 9.9 × 5.6 μm with SD length 0.58 μm and width 0.26 μm, Q value 5–95 % percentile range 1.63–1.93, with median 1.78 and av 1.78 with SD 0.09; spore size based on 22 collections medians 9.0–10.8 × 4.9–6.0 μm and av 9.1–10.8 × 4.9–6.0 μm with SD length 0.40–0.99 μm and width 0.18–0.55 μm, Qav 1.61–2.02. Basidia cylindrical to clavate and 4-spored, 21–30 × 5.5–9.1 μm, with av 22–27 × 6.6–8.2 μm and basidium Qav in the range 2.8–4.2. Pleurocystidia not found, although cheilocystidia sometimes found behind the lamella edge. Cheilocystidia capitate-stipitate or clavate-stipitate, occasionally slightly swollen towards the base (capitate-lageniform or clavate-lageniform) and almost always with apical thickening, occasionally septate and rarely with median thickening; width of apex holotype 5–95 % percentile range 7.5–11.9 μm, with median 9.7 μm and av 9.6 μm with SD 1.25 μm; across 22 collections median 8.2–11.4 μm and av 8.3–11.4 μm; with n ≥ 20 selected cheilocystidia of 22 collections the 5–95% percentile ranges are 35–76 × 6.5–14.1 × 3.1–6.0 × 2.4–6.6 μm while the averages are 44–63 × 8.3–11.4 × 4.0–4.9 × 3.3–5.4 and 47 × 9.6 × 4.3 × 4.4 μm av for the holotype. The av cheilocystidia ratios for the 22 collections were: A/M = 1.90–2.86; A/B = 2.02–3.38; B/M = 0.77–1.17. Caulocystidia resemble cheilocystidia, up to 11 μm wide at the apex but on average shorter than the cheilocystidia. Pileipellis is an ixocutis with a relatively thick epicutis 100–135 μm, embedded hyphae up to 5–6 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis pale yellow and made up of cylindrical to isodiametric elements. Trama below the subcutis contains thick sausage shaped elements up to 15 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — Hebeloma helodes is widespread across northern and central Europe, but as yet we have no confirmed records of this taxon in southern Europe. Also, we have not yet seen any confirmed records from outside of Europe. It has been recorded with a variety of deciduous trees including Alnus, Betula, Corylus, Fagus, Populus, Quercus and Salix spp. However, we suspect that it is not mycorrhizal with Alnus as we have no records of it growing with Alnus when no other trees were present, nor has it, to our knowledge, ever been found in studies of mycorrhiza on Alnus roots. Similarly, although some records include Picea and Pinus, again all of these records also record a deciduous tree close by and we suspect that the mycorrhizal association is with the deciduous tree. The species is found on both calcareous and acid ground, often in sandy or wet places. Habitats are often described as grassy and include deciduous and mixed woodland, often on the side of a path or road.
Additional specimens examined. BELGIUM, prov. Namur, on the road between Voneche and Ave-et-Auffe, Hainaut (c. N50.10 E5.14, alt. c. 180 m) association not recorded, 4 Oct. 1982, J. Boekhout L 0490428, database record HJB12905; prov. Brabant-Wallonia, Bois de Lauzelles, Ottignies (c. N50.6784 E4.5999, alt. c. 110 m) scattered on wet soil in broadleaf woodland with Alnus sp. and Betula sp., 13 Sept. 2003, D. Ghyselinck HJB8816; prov. Luxembourg, Wilbauroche (N49.8133500 E5.2733000, alt. c. 310 m) in litter in broadleaf woodland with Corylus avellana and Fagus sylvatica, 17 Sept. 2004, H. Beker HJB10606. – ENGLAND, South Lancashire, Ainsdale (N53.59273 W3.054548, alt. c. 0 m) scattered in sand on dune with Salix repens, 22 Sept. 2002, H. Beker HJB9266; Shropshire, Poles Coppice (N52.63748 W2.90287, alt. c. 205 m) on grassy soil in broadleaf woodland under Salix sp., 17. Oct. 2002, T. Kirk HJB8181; East Kent, Ashdown Forest (c. N50.8852 E0.033, alt. c. 160 m) on grassy soil, association not recorded, 18 Sept. 2004, A. Andrews HJB10680; South West Yorkshire, Burnt Stones Pasture (c. N53.3772 W1.5505110, alt. c. 265 m) in litter with Salix cinerea, 18 Oct. 2004, C. Hobart, P. Ardron HJB10731. – FAROE ISLANDS, Streymoy, Torshavn, at Foroya Natturugripasavn (c. N62.0167 W6.783, alt. c. 60 m) with Salix phylicifolia, 3 Aug. 1988, J. Vesterholt F560, duplicate HJB10726. – FINLAND, Etelä-Häme, Tampere, Peltolammi (c. N61.44 E23.74, alt. c. 100 m) in mixed woodland with Alnus sp., Betula sp., Picea sp. and Salix sp., 20 Aug. 1992, U. Söderholm US1993, duplicate HJB10727. – FRANCE, Normandy, Foret d’Ecouves (N48.55745 W0.0832667, alt. c. 350 m) in damp litter in a mixed woodland on calcareous soil with Picea sp., Pinus sp. and Quercus sp., 6 Oct. 2006, M. Beker HJB11698; Jura, Lac des Rouges Truites (c. N43.36252 E5.59531, alt. c. 940 m) in mixed woodland with Salix sp., 26 Sept. 1996, D. Aanen WBS 9655, database record HJB12520; Puy-de-Dome, lower lake at La Godivelle (N45.382653 W2.92516, alt. c. 1200 m) with Salix cinerea and S. pentandra, 18 Sept. 1998, G. Corriol, GC98091808, duplicate HJB12930. – NETHERLANDS, Drenthe, Beilen, Holthe, Wilgen Bosje (c. N52.84 E6.56, alt. c. 20 m) in wet Sphagnum with Salix aurita and S. cinerea, 1 Oct. 1996, D. Aanen WBS 9660, database record HJB12521; Drenthe, Beilen, Holthe, Griendje (c. N52.50267 E6.32319, alt. c. 15 m) collected with Salix aurita and S. cinerea, 1 Oct. 1996, D. Aanen WBS 9661, database record HJB12524; Drenthe, Beilen, lieving (c. N52.5124 E6.3040, alt. c. 10 m) on wet soil under Salix aurita and S. cinerea, 1 Oct. 1996, D. Aanen WBS 9664, database record HJB12525; Groningen, Drachten, Boerakker (c. N53.187 E6.329, alt. c. 0 m) with Populus sp. 18 Oct. 1996, D. Aanen WBS 9696, database record HJB12539. – NORWAY, Luster, S. of Fortun (c. N61.44 W7.45, alt. c. 50 m) on soil with Picea sp. and Salix sp., 8 Sept. 2000, J. Vesterholt JV00-277, duplicate HJB10725. – POLAND, open ground between Bialowieza Park and Bialowieski Park Narodowy (N52.7100 W23.84479, alt. c. 150 m) on grassy wet acid soil in broadleaf woodland under Betula sp., 20 Sept. 2008, I. Kałucka, H. Beker HJB12634; Jelonka Reserve Plot 67 (N52.595 E23.359, alt. c. 180 m) on soil in mixed woodland under Pinus sylvestris, Populus tremula and Salix sp., 4 Oct. 1995, I. Kałucka LOD 19484, database record HJB13151. – SCOTLAND, Orkney, Ravie Hill Plantation (c. N59.1056 W3.2867, alt. c. 30 m) in litter in conifer woodland with Pinus contorta, 22 Sept. 1990, T. Eggeling E 00159158, database record HJB11906. – WALES, Denbigh, Erddig (N53.01608 W3.000195, alt. c. 80 m) in litter in broadleaf woodland under Fagus sylvatica, 5 Oct. 2002, H. Beker HJB8115.
Notes — Given the shape of its cheilocystidia, H. helodes clearly belongs to H. subsect. Denudata. The species most likely corresponds to ICG12 of Aanen & Kuyper (1999). We have no confirmed records of this taxon in arctic or alpine habitats although it appears closely related to H. aurantioumbrinum, which does exist in these habitats. It can be readily distinguished from the other members of this subsection that grow in lowland areas based on the number of lamellae, 30–60, the small spore length, < 11 μm, the large average width of the cheilocystidium apex, > 8 μm, and the regular apical thickening of the cheilocystidium. It has probably often been confused with H. pusillum, which is also a small Hebeloma sp., but H. pusillum is confined to Salix, often has < 30 full length lamellae, has a more fragile stature and usually a rather darker centre to the pileus. It also has significantly longer spores, on average. Hebeloma luteicystidiatum also has fewer lamellae and longer spores and is usually somewhat smaller. Hebeloma aurantioumbrinum is rarely found in non alpine/arctic habitats, but in any case can be distinguished microscopically because its cheilocystidia very rarely have any sign of apical thickening. It also usually has a much more brightly coloured pileus.
As shown in Fig. 1 and 2, all loci tested are suitable for distinguishing H. helodes from species other than H. aurantioumbrinum. Hebeloma helodes cannot be distinguished molecularly from H. aurantioumbrinum based on ITS, V6 or V9, but either of the coding genes RPB2 or MCM7 will be suitable for the identification of H. helodes.
Hebeloma louiseae Beker, Vesterh. & U. Eberh., sp. nov. — MycoBank MB809908; Fig. 5d, 18, 19
Fig. 18.
Hebeloma louiseae (BR-MYCO 173982-61, holotype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
Fig. 19.
Hebeloma louiseae (BR-MYCO 173982-61, holotype). a, b. Spores ×1 600 in Melzer’s reagent or 5 % KOH, respectively; c, d. spore ornamentation ×1 600 in Melzer’s reagent or 5 % KOH, respectively; e, f. cheilocystidia ×1 000 in 5 % KOH; g. cheilocystidia ×500 in 5 % KOH; h. basidium ×1 000 in 5 % KOH; i. caulocystidia ×500 in 5 % KOH; j. cutis ×80 in 5 % KOH. — Scale bars: 10 μm, in j. 100 μm.
Etymology. To mark the support Louise Beker has provided to this entire project not only with her time but also travelling to remote places in search of Hebeloma spp.
Type. SVALBARD, Ossian near Ny Ålesund (N78.9260500 E12.4542667, alt. c. 3 m) on mossy soil with Salix polaris, 17 Aug. 2007, M.L. Beker, H. Beker holotype BR BR-MYCO 173982-61; isotypes C C-F-90149, HJB12019.
Diagnosis — Hebeloma louiseae was found in arctic conditions in Svalbard and is most similar to H. minus, from which it differs by the less prominent spore ornamentation - O1 or O2 and at most a few spores O3. It differs from other taxa of H. subsect. Denudata occurring in arctic conditions by its small number of complete lamellae (< 40) in combination with an average cheilocystidium apex width of ≥ 9 μm.
Basidiomes usually in scattered groups. Pileus up to 15 mm diam, convex or plano-convex to broadly umbonate; surface dry or slightly viscid, neither hygrophanous nor striate, sometimes with a slight pruinose layer; cuticle colour clay buff to Isabella sometimes with a thin paler margin; pileus margin straight, sometimes crenulate and slightly involute even in fully grown basidiomes. Lamellae emarginate, quite widely spaced (L = 30–38); colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, paler than lamella surface; droplets on the lamellae edge were not seen; lamellules sparse. Stipe central, cylindrical sometimes slightly clavate or tapering towards the base, (8–)9.5–21.5(–25) × 2–3(–3.5) mm; white or alutaceous, sometimes discolouring brown near the base when handled; surface dry, with fine fibrils and pruina over the length of the stipe. Cortina not observed. Flesh rather thin, cream or pale brown, discolouring slightly when bruised. Smell raphanoid or absent. Taste not recorded. Spore deposit greyish brown.
Spores amygdaloid to limoniform, with small apiculus and rounded at the end opposite the apiculus, with a distinct thinning of the spore wall and often with a pronounced papilla, guttulate with one or more oily drops, almost smooth to very weakly ornamented, with no sign of loosening perispore and dextrinoidity ranging from completely indextrinoid to weakly dextrinoid (O1,O2; P0; D0,D1); spore colour under the microscope yellow brown to brown; spore size based on n = 69 spores of the holotype, 5–95 % percentile range 11.6–14.4 × 6.9–8.6 μm, with median 12.7 × 7.7 μm and av 12.8 × 7.7 μm with SD length 0.82 μm and width 0.53 μm, Q value 5–95 % percentile range 1.50–1.80, with median 1.67 and av 1.66 with SD 0.1; spore size based on 3 collections medians 12.2–12.7 × 7.5–7.7 μm and av 12.3–12.8 × 7.5–7.7 μm with SD length 0.53–0.82 μm and width 0.36–0.53 μm, Qav 1.63–1.66. Basidia cylindrical to clavate and 4-spored, 28–56 × 8.6–11.6 μm, with av 33–48 × 9.5–10.5 μm. Pleurocystidia not found. Cheilocystidia clavate-stipitate to subcapitate-stipitate, occasionally slightly swollen towards the base and occasionally septate, some apical thickening, sinuate or bifurcate; width of apex holotype 5–95 % percentile range 7.9–12.3 μm, with median 9.7 μm and av 9.9 μm with SD 1.48 μm; across 3 collections median 9.0–9.8 μm and av 9.0–9.9 μm; with n ≥ 20 selected cheilocystidia of 3 collections the 5–95 % percentile ranges are 37–76 × 7.1–12.7 × 3.5–6.5 × 4.0–6.8 μm while the averages are 49–59 × 9.0–9.9 × 4.4–5.4 × 4.8–5.6 and 57 × 9.9 × 5.4 × 4.9 μm av for the holotype. The av cheilocystidia ratios for the 3 collections were: A/M = 2.01–2.42; A/B = 1.99–2.25; B/M = 0.92–1.24. Caulocystidia resemble cheilocystidia, up to 75 μm long and 12 μm wide at the apex. Pileipellis is an ixocutis with a medium thick epicutis up to a maximum of 100 μm and embedded hyphae up to 6 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis orange brown and made up of cylindrical to isodiametric elements. Trama below subcutis contains larger thick sausage shaped elements up to 15 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — All 3 collections of H. louiseae have been collected in Svalbard in association with Salix polaris. Two of the collections have been on coastal shingle, not far from the water’s edge but the third collection was from a grazed scrub area 4–5 km from the sea.
Additional specimens examined. SVALBARD, Endalen near Longyearbyen (N78.1940667 E15.7892500, alt. c. 28 m) on soil in grazed valley with Salix polaris, 13 Aug. 2007, J. Sandmo HJB11984; Ossian near Ny Ålesund (N78.9259500 E12.4541167, alt. c. 0 m) on grazed scrub with Salix polaris, 17 Aug. 2007, M.L. Beker, H. Beker HJB12023.
Notes — Hebeloma louiseae has most likely not been included in the intercompatibility tests of (Aanen & Kuyper 1999) and is currently only known from Svalbard, but it has been collected in two sites some 110 km apart. It is likely that it is Salix-specific. The cheilocystidia place this species within H. subsect. Denudata. Given its arctic habitat, small size and very wide weakly ornamented spores this species is easily distinguished from other members of H. subsect. Denudata. While it is possible that this species only exists on Svalbard it is likely that it could be found in other arctic habitats, but its size and camouflaged appearance may mean that it is often overlooked. Also it may in the past have been confused with other small arctic/alpine Hebeloma spp.
Hebeloma louiseae is not only monophyletic, but also receives (high) bootstrap support in four out of five tested loci. Only V9 cannot distinguish it from H. minus. Although the name H. crustuliniforme has traditionally been applied to collections of medium and large sporocarps (Vesterholt et al. 2014), typically growing outside the arctic and alpine habitats, in evolutionary terms, the H. crustuliniforme complex also includes small, arctic/alpine taxa. The analyses of V6, V9 and RPB2 suggest that H. louiseae belongs to the H. crustuliniforme complex. This is contradicted by its placement in the ITS phylogram.
Hebeloma luteicystidiatum Beker, Vesterh. & U. Eberh., sp. nov. — MycoBank MB809909; Fig. 5e, 20, 21
Fig. 20.
Hebeloma luteicystidiatum (BR-MYCO 166233-72, holotype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
Fig. 21.
Hebeloma luteicystidiatum (BR-MYCO 166233-72, holotype). a, b. Spores and spore ornamentation ×1 600 in Melzer’s reagent; c. spore with loosening perispore ×1 600 in 5 % KOH; d, e. spores and spore ornamentation ×1 600 in 5 % KOH; f. cheilocystidia ×1 600 in Melzer’s reagent; g. cheilocystidia ×1 000 in Melzer’s reagent; h. basidium ×1 000 in 5 % KOH; i. cheilocystidia at gill edge ×500 in 5 % KOH; j. trama below cutis ×500 in 5 % KOH. — Scale bars: 10 μm.
Etymology. From luteus - yellow and cystidiatus – having cystidia, to emphasise the thick apical wall that sometimes looks yellow under the microscope.
Type. BELGIUM, prov. Limburg, Houthalen (N51.0154833 E5.3518667, alt. c. 45 m) in wet boggy ground next to slagheap with Salix sp., 22 Oct. 2006, J. Volders holotype BR BR-MYCO 166233-72 (VJ06095); isotypes C C-F-90150, HJB11837.
Diagnosis — Based on its cheilocystidium shape, H. luteicystidiatum is a member of H. subsect. Denudata. The most distinctive and constant character of the species are the thick walls of the cheilocystidium apices. With its small stature and 2-coloured pileus, H. luteicystidiatum is similar to H. pusillum. From the latter it can be separated based on the pileus colour, which may be dark, but not dark brick, and the cystidia of H. pusillum do not have cheilocystidia with wall thickening at the apex. Macroscopically, H. helodes is similar in terms of coloration, but is larger, has more crowded lamellae and shorter spores.
Basidiomes solitary or in scattered groups. Pileus 6–15 mm diam, convex, slightly tacky when moist but never hygrophanous, at high magnification the pileus has a slightly felty look; cuticle colour in the central region ochre to honey or rusty-coloured to dark brown but becoming paler towards the margin which may be anywhere from buff to cream to white; pileus margin usually straight, occasionally involute or eroded with age. Lamellae emarginate, almost free, distant (L = 21–26) with a maximum depth of 2.5 mm; colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, significantly paler than lamella surface; droplets normally visible on the lamella edge usually by the naked eye but certainly with a × 10 lens; lamellules occasional. Stipe central, cylindrical usually stuffed, rarely hollow, 12–30 × 1.0–2.5 mm usually with strong brown discolouration towards the base of the stipe; surface dry, pruinose to floccose. Cortina not observed. Flesh rather thin, whitish but slightly more coloured in the base of the stipe. The whole basidiome has a very slender and fragile appearance with the stipe Q (ratio of stipe length to stipe width) in excess of 12. Smell raphanoid, sometimes absent. Taste not recorded. Spore deposit colour not recorded. Exsiccata fragile and brittle often dark, sometimes blackening.
Spores amygdaloid, with small apiculus and rounded at the end opposite the apiculus, with some thinning of the spore wall, sometimes with a papilla, sometimes guttulate with one or more oily drops, from almost smooth to normally weakly ornamented, sometimes with slightly loosening perispore in a few spores and weakly but sometimes distinctly dextrinoid (O1,O2; P0,P1(P2); D1,D2); spore colour under the microscope yellow brown to brown; spore size based on n = 53 spores of the holotype, 5–95 % percentile range 10.3–13.0 × 5.8–6.9 μm, with median 11.6 × 6.4 μm and av 11.6 × 6.4 μm with SD length 0.80 μm and width 0.32 μm, Q value 5–95 % percentile range 1.63–2.01, with median 1.85 and av 1.84 with SD 0.12; spore size based on 4 collections medians 11.6–11.9 × 6.1–6.4 μm and av 11.6–11.9 × 6.1–6.5 μm with SD length 0.59–0.80 μm and width 0.31–0.36 μm, Qav 1.80–1.95. Basidia cylindrical to clavate and 4-spored, 25–39 × 6.4–10.1 μm, with av 28–34 × 7.1–9.4 μm and basidium Q av in the range 3.3–4.7. Pleurocystidia not found. Cheilocystidia, clavate-stipitate or spathulate-stipitate, occasionally subcapitate-stipitate or slightly swollen towards the base (subcapitate-lageniform or clavate-lageniform), always with distinctive thickening of the apex, so much so that the apex sometimes appears yellow under the microscope, occasionally septate and rarely with median thickening; width of apex holotype 5–95 % percentile range 7.2–11.8 μm, with median 9.1 μm and av 9.3 μm with SD 1.45 μm; across 4 collections median 8.6–9.7 μm and av 8.8–9.9 μm; with n at least 20 selected cheilocystidia of 4 collections the 5–95 % percentile ranges are 37–77 × 6.7–12.6 × 3.0–5.7 × 2.7–6.1 μm while the averages are 50–62 × 8.8–9.9 × 4.0–4.3 × 4.0–4.9 and 50 × 9.3 × 4.0 × 4.9 μm av for the holotype. The av cheilocystidia ratios for the 4 collections were: A/M = 2.27–2.77; A/B = 2.21–2.68; B/M = 0.98–1.22. Caulocystidia resemble cheilocystidia, up to 90 × 10 μm wide at the apex. Pileipellis is an ixocutis with a thin epicutis 50–60 μm, embedded hyphae up to 5 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis orange brown and made up of cylindrical to isodiametric elements. Trama below subcutis contains ellipsoid, cylindrical and angular elements up to 20 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — All records of H. luteicystidiatum are in association with Salix with which it clearly has a mycorrhizal relationship. Furthermore, all collections are from wet boggy areas, usually in Salix thickets in woodland.
Additional specimens examined. BELGIUM, prov. Limburg, Houthalen (N51.0154833 E5.3518667, alt. c. 45 m) in wet boggy ground next to slagheap with Salix sp., 31 Oct. 2007, H. Beker HJB121740. – FRANCE, Yvelines, St-Remy-les-Chevreuse, Bois de Champfailly (N48.72208 E2.0650130, alt. c. 100 m) on boggy wet ground under Alnus sp. and Salix sp., 12 Sept. 1999, G. Corriol GC99081202, duplicate HJB12936. – NETHERLANDS, Amsterdam (c. N52.22 E4.53, alt. c. 30 m) collected with Salix sp., 9 Sept. 1995, D. Aanen WBS 9501, database record HJB12462.
Notes — Given the shape of its cheilocystidia, H. luteicystidiatum clearly belongs to H. subsect. Denudata. The species most likely corresponds to ICG6 of Aanen & Kuyper (1999). It certainly appears to be restricted to Salix and occurs on wet soil, usually in Salix thickets. The basidiomes are very small and we suspect it has either tended to be overlooked or confused with H. pusillum on account of its small slender appearance and 2-coloured cap. However, it can easily be separated from H. pusillum, macroscopically because of the centre which while quite dark is not the distinctive dark brick colour of H. pusillum and microscopically because H. pusillum never has a consistently thickening apex to its cheilocystidia. Hebeloma helodes has pileus colours more like H. luteicystidiatum but is a larger mushroom and while its cheilocystidia also have a thickening apex, its spores are significantly shorter and H. helodes also has rather more crowded lamellae.
Hebeloma luteicystidiatum is molecularly distinct and its clade receives high bootstrap support in all loci apart from RPB2 for which only a single sequence could be obtained. The species is not part of the H. crustuliniforme complex.
Hebeloma lutense Romagn., Bull. Trimestriel Soc. Mycol. France 81: 342. 1965. — MycoBank MB331750; Fig. 22, 23
Fig. 22.
Hebeloma lutense (P 59.232, holotype). a, b. Spores and spore ornamentation × 1 600 in 5 % KOH; c, d. spores and spore ornamentation × 1 600 in Melzer’s reagent; e. cheilocystidia × 1 000 in Melzer’s reagent; f. cheilocystidia × 500 in Melzer’s reagent; g–i. basidia × 1 000 in Melzer’s reagent; i. subcutis × 500 in 5 % KOH; j. epicutis hyphae × 500 in 5 % KOH. — Scale bars: 10 μm.
Fig. 23.
Hebeloma lutense (P 59.232, holotype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
Type. FRANCE, Yvelines, Forêt de Rambouillet (S.-et.O.), étang de la Tour (c. N48.65 E1.88, alt. c. 175 m) at the edge of a lake amongst Phragmites, Carex, Hydrocotyle under mixed Salix sp. and Betula sp., 11 Oct. 1959, H. Romagnesi (holotype PC 59.232, database record HJB1000253; isotype L 0054088, database record HJB1000011).
Basidiomes usually in scattered groups or sometimes caespitose. Pileus 15–58 mm diam, convex, sometimes umbonate, slightly viscid, tacky when moist but never hygrophanous; cuticle colour often almost zonate with the centre from yellow brown to cinnamon to chestnut or even dark brick, sometimes then with a paler but still strongly coloured zone and finally pinkish buff to cream to almost white near the margin; pileus margin usually straight, sometimes involute and occasionally slightly scalloped. Lamellae emarginate, moderately spaced (L = 32–58) with a maximum depth of 5–10 mm; colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, paler than lamella surface; droplets normally visible on the lamella edge even with the naked eye; lamellules frequent. Stipe central, sometimes cylindrical but more often clavate and occasionally even subbulbous, (15–)22.5–90 × 3–11 mm and up to 18 mm at the base, stuffed when young but later hollow and sometimes with a superior wick; white or alutaceous, often with some brown discolouration in older basidiomes; surface dry, pruinose to floccose particularly towards the apex. Cortina not observed. Flesh rather thick, whitish but slightly more coloured in the base of the stipe. Smell raphanoid, sometimes with hint of cocoa, rarely absent. Taste raphanoid to bitter. Spore deposit brownish olive to umber.
Spores amygdaloid, with small apiculus and rounded at the end opposite the apiculus, with a distinct thinning of the spore wall, occasionally with a weak papilla, guttulate with one or more oily drops, from almost smooth to weakly ornamented but more usually distinctly ornamented, usually with some sign of loosening perispore in a few spores and often in many spores and weakly but distinctly dextrinoid ((O1)O2,O3; (P0)P1,P2; D1,D2); spore colour under the microscope yellow brown to brown; spore size based on n = 57 spores of the holotype, 5–95 % percentile range 9.2–11.7 × 5.5–6.6 μm, with median 10.6 × 6.0 μm and av 10.6 × 6.1 μm with SD length 0.73 μm and width 0.34 μm, Q value 5–95 % percentile range 1.62–1.88, with median 1.74 and av 1.74 with SD 0.9; spore size based on 22 collections medians 9.4–11.7 × 5.2–6.3 μm and av 9.4–11.7 × 5.4–6.4 μm with SD length 0.46–1.08 μm and width 0.24–0.57 μm, Qav 1.63–1.91. Basidia cylindrical to clavate and 4-spored, 27–40 × 5.3–8.9 μm, with av 29–38 × 6.5–8.0 μm and basidium Qav unusually high in the range 4.0–5.7. Pleurocystidia not found. Cheilocystidia clavate or clavate-stipitate, occasionally slightly swollen towards the base (clavate-lageniform) and occasionally with septa, sometimes clamped, or median thickening and almost always with a large number of sinuate cheilocystidia; width of apex holotype 5–95 % percentile range 5.8–9.6 μm, with median 7.7 μm and av 7.7 μm with SD 1.22 μm; across 22 collections median 6.6–7.8 μm and av 6.7–7.9 μm; with n ≥ 20 selected cheilocystidia of 22 collections the 5–95 % percentile ranges are 28–71 × 5.2–10.4 × 2.7–5.7 × 2.4–7.4 μm while the averages are 42–56 × 6.7–7.9 × 3.4–4.5 × 3.5–4.8 and 47 × 7.7 × 4.2 × 4.3 μm av for the holotype. The av cheilocystidia ratios for the 22 collections were: A/M = 1.63–2.39; A/B = 1.58–2.34; B/M = 0.95–1.22. Caulocystidia resemble cheilocystidia, up to 95 μm long and 9 μm wide at the apex. Pileipellis is an ixocutis with a relatively thick epicutis 100–180 μm, embedded hyphae up to 5 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis orange brown and made up of cylindrical to isodiametric elements. Trama below subcutis contains ellipsoid or thick sausage shaped elements up to 15 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — All our records of H. lutense have been collected in the presence of Salix spp. and it would appear likely that this species is confined to Salix. Many of the collections have been from wet or damp environments on acid soil. It is interesting to note that while our description is based on 22 collections of H. lutense, on our database we have 53 records. All of these are from Western Europe (highest longitude is < E11°).
Additional specimens examined. BELGIUM, prov. Hainaut, Maubray (N50. 5306167 E3.4941000, alt. c. 30 m) slagheap, on mossy soil, scattered with Salix sp. and Betula sp., 10 Nov. 2006, P.-A. Moreau HJB11719; prov. Limbourg, Tiewinkel (c. N50.967 E5.233, alt. c. 25 m) scattered on wet acid soil with Salix cinerea, 12 Sept. 2004, A. de Haan AdH04059, duplicate HJB10523; prov. Hainaut, Maubray (N50.5306167 E3.4941000, alt. c. 29 m) on rotten litter in broadleaf woodland on slagheap under Betula sp., Salix sp., 10. Nov. 2006, P.-A. Moreau HJB11726. – DENMARK, WJ, Marbaek plantation north of Esbjerg UTM MG5757 TBU 27 (c. N55.550 E8.310, alt. c. 0 m) on wet soil under Salix sp., 17 Sept. 1995, J. Vesterholt JV95-357, duplicate HJB10876; Naun Sø (c. N56.8 E9.5, alt. c. 40 m) scattered at lakeside with Salix sp., 24 Oct. 1996, T. Læssøe TL4413, duplicate HJB10905. – ENGLAND, Surrey, Bookham Common (c. N51.27 W0.37, alt. c. 100 m) in mixed woodland under Betula sp. and Salix sp., 15 Oct. 1955, P.D. Orton E 00076300, database record HJB12917: this forms part of a collection labelled as H. leucosarx which was mixed and the part that does not represent H. leucosarx as recently selected by Grilli (2007); Surrey, Boldermere (N51.3144100 W0.4517496, alt. c. 30 m) on wet soil in mixed woodland under Salix sp., 28 Sept. 2002, H. Beker HJB8098. – FRANCE, Landes, Mios (N44.5993667 W0.9407333, alt. c. 0 m) in damp litter in a broadleaf woodland with Salix sp., 17 Oct. 2005, H. Beker HJB11328; Alpes-Maritimes, Les Espagnols (N43.5090667 E6.7947833, alt. c. 186 m) on lakeside amongst wet litter in mixed woodland, under Populus sp. and Salix sp., 16 Oct. 2007, H. Beker HJB12122; Nord Pas de Calais, Saint Amand (N50.3976000 E3.4439833, alt. c. 20 m) on wet soil in broadleaf woodland scrub under Salix sp., 10 Nov. 2006, P.-A. Moreau HJB11755; Alpes-Maritimes, Les Espagnols (N43.5090333 E6.7947667, alt. c. 185 m) on litter in mixed woodland under Populus sp., Salix sp., 16 Oct. 2007, P. Cavanagh HJB12126. – NETHERLANDS, Drenthe, Beilen, Wijster, IJsbaantje (c. N52.82 E6.52, alt. c. 10 m) collected in wet Sphagnum with Salix aurita, Salix cinerea and Salix repens, 1 Oct. 1996, D. Aanen WBS 9662, database record HJB12522; Drenthe, Eemsterveld, langs de Drentse Aa (c. N53.15 E6.60, alt. c. 2 m) under Salix sp., 10 Oct. 1995, D. Aanen WBS 9571, database record HJB12808; Drenthe, Beilen, Wijster ijsbaantje (c. N52.82 E6.52, alt. c. 10 m) collected in acid wet soil with Sphagnum and Salix repens, 1 Oct. 1996, D. Aanen WBS 9663, database record HJB12523. – SCOTLAND, Orkney (c. N59.64959 W3.0094460, alt. c. 0 m) in dune in sandy soil with Salix repens, 21 Aug. 2002, A. Andrews HJB9819; Loch Loy (c. N57.58 W3.85, alt. c. 10 m) in wet mixed woodland under Betula sp. and Salix sp., 25 Sept. 1955, P.D. Orton K K(M)52712, database record HJB1000001: this forms part of the holotype collection of H. leucosarx which was mixed and the part not selected as typus for H. leucosarx by Grilli (2007); Easterness, Bogach (N57.1659300 W3.8482160, alt. c. 235 m) in bog on pathside with Salix sp., 22 Aug. 2005, S. Huhtinen SH05/44, duplicate HJB11168. – SPAIN, Castilla y Leon, Rio Cerneja (N43.1177667 W3.4608833, alt. c. 835 m) in acid litter with Alnus glutinosa, Quercus pyrenaica and Salix atrocinerea, 21 Oct. 2005, I. Olariaga Ibarguren HJB11344, duplicate JV05-602; Castilla y Leon, Rio Cerneja (N43.1226167 W3.4653500, alt. c. 825 m) caespitose in acid grassy soil with Alnus glutinosa, Quercus pyrenaica and Salix atrocinerea, 21 Oct. 2005, I. Olariaga Ibarguren HJB11365, duplicate JV05-634: note this collection was almost sterile; Castilla y Leon, Rio Cerneja (N43.1226167 W3.4653500, alt. c. 827 m) on grassy soil in mixed woodland under Pinus sylvestris, Salix atrocinerea, 21. Oct. 2005, I. Olariaga Ibarguren JV05-621, HJB11356; Castilla y Leon, Rio Cerneja (N43.1226167 W3.4653500, alt. c. 827 m) in mixed woodland under Alnus glutinosa, Quercus pyrenaica, 21 Oct. 2005, H. Beker HJB11363, duplicate JV05-631.
Notes — Given the shape of its cheilocystidia, H. lutense clearly belongs to H. subsect. Denudata. The species most likely corresponds to ICG9 of Aanen & Kuyper (1999). Its sinuate cheilocystidia and its consistently long thin basidia distinguish it from the other members of this subsection. Sinuate cheilocystidia do occur in other species, for example H. eburneum, but in our experience there is no other species where sinuate cheilocystidia occur so frequently and is such a consistent character. Similarly other species in this subsection can have quite long narrow basidia but again this taxon has them consistently. The basidium Q for this taxon ranges from 4.0–5.7. The combination of these sinuate cheilocystidia and the high average Q of the basidia unambiguously define this taxon. Additionally, the number of complete lamellae < 60 and the average cheilocystidium apex width of < 8 μm, means it is highly unlikely to miss-determine this taxon.
The records on our database for H. lutense are confined to Salicaceae and to Western Europe, i.e. the most easterly collection is from Denmark. We suspect that an exclusive mycorrhizal association with Salix is correct. With regard to the geographical distribution we must await more confirmed records. While we do not have sufficient data to suggest that this species is restricted to Western Europe, it certainly appears more common in this area.
Hebeloma lutense is monophyletic and receives high bootstrap support in all tested loci apart from the ITS. The placement of the incomplete sequence of the isotype of H. lutense outside the H. lutense clade of the ITS result can be explained by missing data. The placement of a single H. alpinum collection (HJB11997; its ITS sequence has been confirmed by repetition and its V6 sequence is clearly not H. lutense) in the clade that otherwise only contains sequences from H. lutense collections only shows how similar the ITS is in this subsection. However, normally an ITS2 sequence of H. lutense should be sufficient to identify this species, too.
Hebeloma minus Bruchet, Bull. Mens. Soc. Linn. Lyon 39, 6 (Suppl.): 126. 1970. — MycoBank MB314960; Fig. 24, 25
Fig. 24.
Hebeloma minus (LY BR69-15, holotype). a, b. Spores and spore ornamentation × 1 600 in Melzer’s reagent; c, d. spores and spore ornamentation × 1 600 in 5 % KOH; e–h. cheilocystidia ×500 in 5 % KOH; i, j. basidia ×500 in 5 % KOH; k. trama below cutis ×500 in 5 % KOH. — Scale bars: 10 μm.
Fig. 25.
Hebeloma minus (LY BR69-15, holotype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
Type. FRANCE, Savoie, Lac des Assiettes, Col de la Vanoise (c. N45.389 E6.792, alt. c. 2500 m) on alpine scrub, in calcareous dry lake bed with Salix herbacea, 11 Sept. 1969, G. Bruchet, LY BR69-15, database record HJB1000065.
Basidiomes usually in scattered groups. Pileus 9–31 mm diam, convex to umbonate, slightly tacky when moist and sometimes hygrophanous; cuticle colour from dark pinkish buff or Isabella to brownish olive, greyish brown or umber sometimes unicoloured but sometimes paler towards the margin which may be pinkish buff, occasionally pruinose (givré) especially after frosting; pileus margin usually straight, sometimes involute particularly in young basidiomes. Lamellae emarginate to adnate, relatively distant (L = 30–34) with a maximum depth of 4–5 mm; colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, significantly paler than lamella surface; droplets normally visible on the lamella edge usually by naked eye but certainly with a × 10 lens, rarely absent; lamellules occasional. Stipe central, cylindrical or slightly clavate, rarely bulbous, becoming hollow with age, 10–40 × 1–8 mm and up to 10 mm at the base rarely with some weak discolouration towards the base of the stipe; surface dry, pruinose to floccose, especially towards the apex. Cortina not observed. Flesh medium thick, pale buff. Smell raphanoid, sometimes weak. Taste mild, occasionally weakly bitter or weakly raphanoid. Spore deposit brownish olive. Exsiccata fragile and brittle.
Spores amygdaloid or limoniform, with small apiculus and rounded at the end opposite the apiculus, with some thinning of the spore wall, and usually with a papilla, sometimes guttulate with one or more oily drops, from weakly to distinctly ornamented, sometimes with slightly loosening perispore in a few to many spores and weakly but sometimes distinctly dextrinoid (O2,O3; P0,P1,P2; D1(D2)); spore colour under the microscope yellow to pale brown; spore size based on n = 64 spores of the holotype, 5–95 % percentile range 11.0–13.9 × 6.1–7.3 μm, with median 12.4 × 6.7 μm and av 12.4 × 6.7 μm with SD length 0.96 μm and width 0.40 μm, Q value 5–95 % percentile range 1.68–2.07, with median 1.84 and av 1.86 with SD 0.12; spore size based on 10 collections medians 11.2–13.1 × 6.3–7.6 μm and av 11.2–13.1 × 6.2–7.7 μm with SD length 0.62–1.12 μm and width 0.32–0.54 μm, Qav 1.61–1.87. Basidia cylindrical to clavate and 4-spored, 27–39 × 8.2–11.5 μm, with av 27–35 × 8.9–10.9 μm and basidium Qav in the range 2.8–3.8. Pleurocystidia not found. Cheilocystidia capitate-stipitate, clavate-stipitate or spathulate-stipitate, occasionally swollen towards the base (capitate-lageniform or clavate-lageniform), sometimes with thickening of the apex, and often appearing bent in the centre (kneeling), occasionally septate; width of apex holotype 5–95 % percentile range 6.7–10.6 μm, with median 9.0 μm and av 8.8 μm with SD 1.40 μm; across 10 collections median 8.9–10.5 μm and av 8.8–10.4 μm; with n ≥ 20 selected cheilocystidia of 10 collections the 5–95 % percentile ranges are 35–67 × 6.3–12.7 × 2.9–6.2 × 2.7–8.6 μm while the averages are 40–55 × 8.8–10.4 × (3.7–)4.2–5.4 × (3–)4.3–5.9 and 55 × 8.8 × 4.2 × 4.6 μm av for the holotype. The av cheilocystidia ratios for the 5 collections were: A/M = 1.99–2.44; A/B = 1.82–3.02; B/M = 0.84–1.24. Caulocystidia resemble cheilocystidia often gregarious, up to 70 × 12 μm wide at the apex. Pileipellis is an ixocutis with an epicutis from thin to medium thick, 40–100 μm, embedded hyphae up to 6.5 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis orange brown and made up of cylindrical to isodiametric elements. Trama below subcutis contains ellipsoid, cylindrical, spherical and sausage-shaped elements up to 30 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — We have records of H. minus both in arctic and alpine locations. In these locations it has been recorded with a variety of Salix shrubs and also with Dryas, specifically Salix herbacea, S. polaris, S. reticulata, S. retusa and Dryas octopetala. We also have one record of this species in a subalpine habitat with Salix caprea. The descriptions include acid, calcareous soil conditions and mossy, wet and exposed ground. As well as the ten European collections of H. minus, on which our description is based, we have also studied one collection from Canada.
Additional specimens examined. France, Jura, Lac des Rouges Truites (c. N46.36252 E5.59531, alt. c. 940 m) under Salix caprea, 22 Sept. 1996, D. Aanen WBS 9630, database record HJB12512. – Iceland, Nordur-Mulasysla, Hamborg, road to Jokladalur (c. N65.050 W14.917, alt. c. 400 m) in mountain heathland under Salix herbacea, 6 Aug. 1993, J. Vesterholt JV93-503, duplicate HJB10865; Nordur-Mulasysla, Hamborg, road to Jokladalur (c. N65.05 W14.917, alt. c. 400 m) in mountain heathland under Salix herbacea, 6 Aug. 1993, J. Vesterholt JV93-506, duplicate HJB10866; Valavatn (N64.8665500 W23.5597167, alt. c. 301 m) on rotten litter in scrub under Salix herbacea, 29 July 2005, H. Beker, M.L. Beker HJB11079. – Svalbard, Vestspitsbergen, Van Mijenfjorden, Sveagruva, Kylan W-puolella, Tuore sammaleinen tunturiniitty (N77.8848340 E16.9745880, alt. c. 300 m) on mossy, wet soil under Salix sp., 20 July 1969, S. Eurola, A. Hakala OULU EO20.7.69; F067388, database record HJB13577; Colesdalen (N78.1153333 E15.0278667, alt. c. 30 m) on soil in grazed grassland under Salix polaris, 10 Aug. 2007, H. Beker, M.L. Beker HJB11945; Ekmanfjorden (N78.6240500 E14.8289167, alt. c. 8 m) on exposed soil in maritime coastal scrub under Salix polaris, 15 Aug. 2007, H. Beker, M.L. Beker HJB12007. – Switzerland, Tessin, Val Bedretto, Col de Gries, TI (c. N46.4667 E8.3740, alt. c. 2190 m) Schneetälchen, Fleckenmergel, under Dryas octopetala, Salix herbacea, Salix reticulata, Salix retusa, 28 Aug. 1988, E. Horak ZT 4113, duplicate HJB12568; Oberaar (N46.5485667 E8.2768333, alt. c. 2312 m) on soil in wasteland under Salix sp., 10 Aug. 2005, H. Beker, M.L. Beker HJB11107.
Notes — Given the shape of its cheilocystidia, H. minus clearly belongs to H. subsect. Denudata. The species most likely corresponds to ICG7 of Aanen & Kuyper (1999). It certainly appears to be restricted to Salix (and possibly Dryas) and while it appears to be predominantly an arctic/alpine species it can occur in subalpine habitats and presumably subarctic habitats. While at present we only have 11 recorded collections of H. minus we would suspect that it is widely distributed throughout the arctic and alpine regions, appearing occasionally in the boreal zone. When we look at the distribution of our collections they are clustered in Iceland, Svalbard and the French/Swiss Alps, plus we have one collection from Canada (not included in our description). Hebeloma minus appears morphologically close to H. alpinum and H. pallidolabiatum. It can be difficult to separate from H. alpinum microscopically but can be separated on macroscopic characters, since H. minus is smaller with a darker coloured pileus and with fewer lamellae than H. alpinum. Hebeloma minus is macroscopically very similar to H. pallidolabiatum but can be separated microscopically using the cheilocystidium ratio A/B which is always > 1.8 for H. minus but < 1.8 for H. pallidolabiatum. With regard to the other alpine/arctic species, it can be distinguished from H. aurantioumbrinum through the average width of the cheilocystidia apex which ≤ 8.5 μm for H. aurantioumbrinum while the average cheilocystidium apex for H. minus is always > 8.5 μm. It can be separated from H. louiseae which has spores O1 or O2 and very rarely O3, while H. minus has many spores O3. In subalpine areas it is most similar to H. salicicola and H. pusillum. But H. pusillum has av spore Q > 1.9 while H. minus av spore Q ≤ 1.9 and H. salicicola has spores D2 or even D3 while H. minus spores are usually D1 and rarely even D2.
As H. alpinum, H. minus is a rather diverse species molecularly. The only single locus in which all available H. minus sequences form a supported monophyletic clade is RPB2. In other single locus analyses, H. minus forms mixed clades with either H. louiseae or H. pallidolabiatum or some of the H. minus sequences are included in the unresolved H. crustuliniforme complex part of the tree.
Hebeloma pallidolabiatum Beker & U. Eberh., sp. nov. — MycoBank MB809910; Fig. 5f, 26, 27
Fig. 26.
Hebeloma pallidolabiatum (BR-MYCO 174908-17, holotype). a, b. Spores and spore ornamentation ×1 600 in 5 % KOH; c, d. spores and spore ornamentation ×1 600 in Melzer’s reagent; e, f. basidia ×1 000 in 5 % KOH; g, h. cheilocystidia ×1 000 in 5 % KOH; i. caulocystidia ×1 000 in 5 % KOH; j. trama below cutis ×500 in 5 % KOH. — Scale bars: 10 μm.
Fig. 27.
Hebeloma pallidolabiatum (BR-MYCO 174908-17, holotype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
Etymology. From pallidus meaning pale and labiatus of the lip, to emphasise the thin pale margin that appears to be a consistent character of this taxon.
Type. SVALBARD, Skansbukta (N78.5156167 E16.0139500, alt. c. 34 m) on soil in coastal scrub under Salix polaris, 14 Aug. 2007, M.L. Beker, H. Beker holotype BR BR-MYCO 174908-17; isotypes C C-F-92312, HJB11992).
Diagnosis — Hebeloma pallidolabiatum belongs, based on its cheilocystidium shape to H. subsect. Denudata. It can be separated from the other small arctic species of this section by the cheilocystidium ratio apex : base (A/B) which is always < 1.8 and the spores, most of which are always quite distinctly ornamented and > 12 μm in length.
Basidiomes in a scattered group. Pileus 12–21 mm diam, convex to broadly umbonate, slightly tacky when moist; cuticle colour 2-coloured, sepia to dark brick in the centre with a thin paler margin; pileus margin straight. Lamellae emarginate, quite distant (L = 30–33); colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, significantly paler than lamella surface droplets usually visible by naked eye; lamellules occasional. Stipe central, cylindrical, 14–24 × 2.0–3.5 mm with some brown discolouration towards the base of the stipe; surface dry, pruinose. Cortina not observed. Flesh rather thin, whitish but slightly more coloured in the base of the stipe. Smell raphanoid. Taste not recorded. Spore deposit Isabella to brownish olive.
Spores amygdaloid, with small apiculus and rounded at the end opposite the apiculus, with some thinning of the spore wall, with a papilla and guttulate, weakly but distinctly ornamented, at least under immersion, with no sign of loosening perispore and at most weakly dextrinoid (O2; P0; (D0)D1,D2); spore colour under the microscope brownish yellow; spore size based on n = 58 spores of the holotype, 5–95 % percentile range 12.0–14.1 × 7.0–8.5 μm, with median 12.8 × 7.5 μm and av 12.8 × 7.6 μm with SD length 0.65 μm and width 0.44 μm, Q value 5–95 % percentile range 1.57–1.80, with median 1.69 and av 1.69 with SD 0.07; spore size based on 2 collections medians 12.8–13.5 × 7.2–7.5 μm and av 12.8–13.5 × 7.2–7.6 μm with SD length 0.65–0.89 μm and width 0.33–0.44 μm, Qav 1.69–1.88. Basidia cylindrical to clavate and 4-spored, 26–40 × 6.6–10.6 μm, with av 27–37 × 7.9–9.8 μm and basidium Qav 3.6–3.9. Pleurocystidia not found. Cheilocystidia clavate-stipitate sometimes slightly swollen towards the base giving an hourglass appearance clavate-lageniform, occasionally with septa (sometimes clamped); width of apex holotype 5–95 % percentile range 6.8–11.5 μm, with median 8.8 μm and av 9.0 μm with SD 1.66 μm; across 2 collections median 8.5–8.8 μm and av 8.6–9.0 μm; with n ≥ 20 selected cheilocystidia of 2 collections the 5–95 % percentile ranges are 48–73 × 6.8–11.5 × 4.1–6.4 × 3.6–7.9 μm while the averages are 58–59 × 8.6–9.0 × 5.1–5.4 × 5.9–6.3 and 59 × 9.0 × 5.1 × 6.3 μm av for the holotype. The av cheilocystidia ratios across the 2 collections were: A/M = 1.61–2.05; A/B = 1.57–1.69; B/M = 1.09–1.24. Caulocystidia resemble cheilocystidia, up to 90 μm long and 11 μm wide at the apex. Pileipellis is an ixocutis with a thin to medium thickness epicutis up to 80 μm, embedded hyphae up to 5.5 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis orange brown and made up of cylindrical to isodiametric elements. Trama below subcutis contains angular elements, sometimes shaped like thick sausages up to 20 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — We only have two collections of this species, both from Svalbard in arctic conditions with dwarf Salix but over 100 km apart. Consequently its habitat is not yet well understood.
Additional specimens examined. SVALBARD, Knudsenheia (N78.9375333 E11.8438500, alt. c. 8 m) on soil in grazed scrubland under Salix polaris, 19 Aug. 2007, M.L. Beker, H. Beker HJB12059.
Notes — Given the shape of its cheilocystidia, H. pallidolabiatum clearly belongs to H. subsect. Denudata. The species has most likely not been included in the intercompatibility tests of Aanen & Kuyper (1999). Of the members of H. subsect. Denudata it is most likely to be confused with the other small arctic/alpine species: H. louiseae, H. minus and H. perexiguum. It can be readily separated from the first two of these on the basis of the cheilocystidium ratio A/B which for this taxon is always < 1.8 whereas it is > 1.8 for both H. louiseae and H. minus. The separation from H. perexiguum is straightforward as the spores for H. pallidolabiatum are more ornamented and longer. Given the small number of collections on which our description is based it is possible that our description is too narrow but until more collections of this taxon are recorded we cannot be sure.
Hebeloma pallidolabiatum is monophyletic in four out of five loci and supported by bootstrap in three of them. Two ITS sequences were obtained from the type collection, of which one is included in a (the) H. pallidolabiatum clade and the other is part of the unresolved H. crustuliniforme complex part of the tree. This provides further evidence that the ITS is less suited for species identification than any of the other loci.
Hebeloma perexiguum Beker, Vesterh. & U. Eberh., sp. nov. — MycoBank MB809911; Fig. 5g, 28, 29
Fig. 28.
Hebeloma perexiguum (BR-MYCO 173979-58, holotype). a, b. Spores and spore ornamentation ×1 600 in Melzer’s reagent; c, d. spores and spore ornamentation ×1 600 in 5 % KOH; e. basidium ×1 000 in 5 % KOH; f. cheilocystidia ×1 000 in 5 % KOH; g. cheilocystidia ×500 in 5 % KOH; h. epicutis hyphae ×1 000 in 5 % KOH; i. caulocystidia ×1 000 in 5 % KOH; j. caulocystidia ×500 in 5 % KOH. — Scale bars: 10 μm.
Fig. 29.
Hebeloma perexiguum (BR-MYCO 173979-58, holotype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
Etymology. From per – very and exiguus – small, to emphasise the small size of this mushroom.
Type. SVALBARD, London near Ny Alesund (N78.9631 E12.05035, alt. c. 5 m) in scrub on site of deserted settlement with Salix polaris, 18 Aug. 2007, M.L. Beker, H. Beker holotype BR BR-MYCO 173979-58; isotype HJB12038.
Diagnosis — Hebeloma perexiguum belongs based on its cheilocystidium shape to H. subsect. Denudata. It can be recognized by the swollen basal part of the cheilocystidia and its small size. It can be recognized from other species of the subsection that are known to occur in arctic/alpine habitats and form small basidiomes, such as H. salicicola by its small number of full length lamellae, which is < 30, from H. minus and H. pallidolabiatum by its low spore ornamentation (O1), from H. aurantioumbrinum by its spore width being > 7 μm and from H. louiseae by its average cheilocystidium apex width of < 9 μm.
Basidiomes in a scattered group. Pileus 7–12 mm diam, convex, slightly tacky when moist; cuticle colour almost unicolour, greyish brown with a thin paler margin; pileus margin straight. Lamellae emarginate, quite distant (L = 24–26); colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, significantly paler than lamella surface droplets not seen on the lamella edge; lamellules occasional. Stipe central, cylindrical, 10–15 × 2.0 mm with some weak brown discolouration towards the base of the stipe; surface dry, pruinose. Cortina not observed. Flesh rather thin, whitish but slightly more coloured in the base of the stipe. Smell absent. Taste not recorded. Spore deposit not recorded. Exsiccata fragile and brittle and pileus blackening.
Spores amygdaloid, with small apiculus and rounded at the end opposite the apiculus, with some thinning of the spore wall, sometimes with a papilla, not guttulate, almost smooth, with ornamentation hardly visible, with no sign of loosening perispore and weakly dextrinoid (O1; P0; D1); spore colour under the microscope brownish yellow; spore size based on n = 56 spores of the holotype, 5–95 % percentile range 9.9–13.4 × 6.4–8.1 μm, with median 11.7 × 7.2 μm and av 11.7 × 7.2 μm with SD length 1.15 μm and width 0.56 μm, Q value 5–95 % percentile range 1.43–1.86, with median 1.64 and av 1.63 with SD 0.12. Basidia cylindrical to clavate and 4-spored, 31–43 × 8.9–10.1 μm, with av 38 × 9.6 μm and basidium Qav 3.9. Pleurocystidia not found. Cheilocystidia spathulate-stipitate or clavate sometimes slightly swollen towards the base giving an hourglass appearance or even appearing lageniform, with some thickening of the apex, occasionally with septa; width of apex holotype 5–95 % percentile range 6.7–10.6 μm, with median 8.2 μm and av 8.5 μm with SD 1.56 μm and overall av dimensions 54 × 8.5 × 4.8 × 6.4 μm av for the holotype. The av cheilocystidia ratios were: A/M = 1.75; A/B = 1.45; B/M = 1.33. Caulocystidia resemble cheilocystidia, up to 75 μm long and 9 μm wide at the apex. Pileipellis is an ixocutis with a very thin epicutis up to 30 μm, embedded hyphae up to 6 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis orange brown and made up of cylindrical to isodiametric elements. Trama below subcutis contains angular elements, sometimes shaped like thick sausages up to 16 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — We only have one collection of this species, growing in arctic conditions with dwarf Salix. Consequently its habitat is not yet well understood.
Notes — Given the shape of its cheilocystidia, H. perexiguum clearly belongs to H. subsect. Denudata. Of the members of H. subsect. Denudata it is the species with the most swollen basal part of the cheilocystidium and may be confused with species from other subsections of H. sect. Denudata, however there is no other arctic/alpine species in H. sect. Denudata, outside H. subsect. Denudata that is so small. Based on just one collection, this species appears to be readily distinguishable from other species in H. subsect. Denudata with which it might be confused. These would most likely be H. louiseae, H. minus, H. pallidolabiatum or small examples of H. aurantioumbrinum or H. salicicola. Based on its small number of full length lamellae, < 30, it is distinguished from alpine forms of H. salicicola. The low ornamentation of the spores will distinguish it from H. minus and H. pallidolabiatum. The wide spores, > 7 μm distinguish it from H. aurantioumbrinum and the cheilocystidium average apex width of < 9 μm distinguishes it from H. louiseae. Given that our description is based on a single collection it is possible that our description is too narrow but until more collections of this taxon are recorded we cannot be sure.
The single H. perexiguum sequence is not included in any species clade for any of the tested loci. Based on the available data it is likely that the species can be recognized by any of the loci used in ML analyses, but its placement among the included taxa varies. In the result of the V9 analysis it is included in a supported clade with H. pusillum (which is highly supported as a species, too), but a sister species relationship with H. pusillum is not indicated by any of the other loci phylograms.
Hebeloma pusillum J.E. Lange, Fl. Agaric Danic. 5 (Taxon. Consp.): iv. 1940. — MycoBank MB275806; Fig. 30, 31
Fig. 30.
Hebeloma pusillum (C JV91-685, epitype). a, b. Spores and spore ornamentation ×1 600 in Melzer’s reagent; c. cheilocystidia x 630 in Melzer’s reagent; d. cheilocystidia ×500 in Melzer’s reagent; e, f. basidia ×1 000 in Melzer’s reagent; g. caulocystidia ×500 in 5 % KOH; h. epicutis hyphae ×500 in 5 % KOH; i. trama below cutis ×500 in 5 % KOH. — Scale bars: 10 μm.
Fig. 31.
Hebeloma pusillum (C JV91-685, epitype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
= Hebeloma pusillum var. longisporum Bruchet, Bull. Mens. Soc. Linn. Lyon 39, 6 (Suppl.): 126. 1970.
= Hebeloma vaccinum var. cephalotum Enderle & Vesterh. in Die Pilzflora des Ulmer Raumes (Ulm): 379. 2004.
Type. DENMARK, Fyn, Langesøskovene S. of Morud (c. N55.45 E10.19, alt. c. 30 m) with Salix sp., Danske Agaricacéer, pl. 460 (aquarelle, C), reproduced in Lange 1938 as pl. 120C; same locality, 16 Oct. 1991, J. Vesterholt, epitypus C JV91-685, database record HJB1000128, selected by J. Vesterholt in Fungi of Northern Europe 3: 82. 2005.
Basidiomes usually in scattered groups. Pileus 5–40 mm diam, convex or applanate, sometimes with a small umbo, often viscid, slightly tacky when moist but never hygrophanous; cuticlecolour cinnamon to sepia to dark brick in the central region and becoming paler towards the margin which may be anywhere from cream to clay coloured, distinctly 2-coloured in older specimens; pileus margin usually straight, occasionally involute when young and sometimes upturned with age. Lamellae emarginate, quite widely spaced (L = 20–38) with a maximum depth of 3–4 mm; colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, significantly paler than lamella surface; droplets normally visible on the lamella edge even with the naked eye; lamellules occasional. Stipe central, cylindrical usually stuffed, rarely hollow, 14–58 × 1.5–2.5(–3.5) mm usually with some brown discolouration towards the base of the stipe; surface dry, pruinose. Cortina not observed. Flesh rather thin, whitish but slightly more coloured in the base of the stipe. The whole basidiome has a very slender and fragile appearance with the av stipe Q (ratio of stipe length to stipe width) usually well in excess of 11. Smell raphanoid, sometimes absent. Taste raphanoid. Spore deposit brownish olive to umber.
Spores amygdaloid or fusoid, with small apiculus and rounded at the end opposite the apiculus, with some thinning of the spore wall, rarely with any papilla, sometimes guttulate with one or more oily drops, from weakly to distinctly ornamented, usually with some sign of loosening perispore in at least a few spores and often in many spores and weakly but sometimes distinctly dextrinoid ((O1)O2,O3; (P0)P1,P2; (DO)D1,D2); spore colour under the microscope yellow brown to brown; spore size based on n = 55 spores of the epitype, 5–95 % percentile range 11.1–13.7 × 5.8–7.0 μm, with median 12.3 × 6.4 μm and av 12.3 × 6.4 μm with SD length 0.87 μm and width 0.48 μm, Q value 5–95 % percentile range 1.79–2.11, with median 1.92 and av 1.93 with SD 0.13; spore size based on 20 collections medians 11.3–13.6 × 5.6–6.7 μm and av 11.4–13.6 × 5.6–6.7 μm with SD length 0.64–1.04 μm and width 0.22–0.55 μm, Qav 1.91–2.22. Basidia cylindrical to clavate and 4-spored, 23–40 × 5.7–10.7 μm, with av 25–35 × 6.8–9.6 μm and basidium Qav in the range 3.0–4.4. Pleurocystidia not found. Cheilocystidia capitate-stipitate, clavate-stipitate or spathulate-stipitate, occasionally slightly swollen towards the base (capitate-lageniform or clavate-lageniform), occasionally with septa and rarely with median thickening, sometimes the cheilocystidia appear conglutinate and tend to break rather than separate; width of apex epitype 5–95 % percentile range 6.1–12.4 μm, with median 8.7 μm and av 9.0 μm with SD 1.96 μm; across 20 collections median 7.9–9.9 μm and av 8.0–10.0 μm; with n ≥ 20 selected cheilocystidia of 20 collections the 5–95 % percentile ranges are 32–97 × 5.6–12.8 × 2.7–6.1 × 2.7–6.8 μm while the averages are 41–70 × 8.0–10.0 × 3.8–4.8 × 3.6–4.9 and 70 × 9.0 × 4.3 × 4.4 μm av for the epitype. The av cheilocystidia ratios for the 15 collections were: A/M = 1.71–2.71; A/B = 1.68–2.52; B/M = 0.94–1.20. Caulocystidia resemble cheilocystidia, up to 75 × 12 μm wide at the apex. Pileipellis is an ixocutis with a thin epicutis 40–80 μm, embedded hyphae up to 6 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis red brown and made up of cylindrical to isodiametric elements. Trama below subcutis contains ellipsoid, cylindrical and thick sausage shaped elements up to 18 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — All our records of H. pusillum record Salix as present. Thus we believe that this species is confined in its mycorrhizal associations to Salix spp. All our records also indicate moist or wet ground, sometimes moss-covered, including records where it has been collected in Sphagnum. The habitat is often described as a Salix thicket but it also occurs on slagheaps or in scrubland. Based on our records H. pusillum is widespread across Central and Western Europe, but we suspect that it is widespread across all of Europe. Although generally not common it can be locally common.
Additional specimens examined. BELGIUM, prov. Hainaut, Maubray (N50. 5306167 E3.4941000; alt. c. 30 m) slagheap, on mossy soil, scattered with Salix sp. and Betula sp., 10 Nov. 2006, P.-A. Moreau HJB11723; prov. Brussels, Hof Musschen (N50.8538167 E4.4458333, alt. c. 53 m) on wet litter in broadleaf woodland scrub under Salix sp., 20 Oct. 2006, H. Beker HJB11716; prov. Hainaut, Maubray (N50.5308500 E3.4940333, alt. c. 29 m) on rotten litter in broadleaf woodland on slagheap under Betula sp., Salix sp., 10 Nov. 2006, P.-A. Moreau HJB11728. – DENMARK, Kogsbøl Mose N. of Højer (c. N56.01 E8.72, alt. c. 15 m) with Salix sp., 23 Sept. 1991, T. Læssøe TL2542, duplicate HJB10941; Snarup Mose SW of Kværndrup (c. N56.16 E10.44, alt. c. 65 m) with Salix sp., 28 Aug. 1993, J. Vesterholt JV93-758, duplicate HJB10715; Søgårds Mose, Fløjstrup Skov S. of Århus (c. N56.08 E10.24, alt. c. 25 m) in boggy soil with Salix sp., 6 Oct. 1991, J. Petersen JV91-525, duplicate HJB10880; region NWJ, Stokholm Mose, north side of Skalleso, NE of Vinderup UTM MH9162 TBU (c. N56.500 E8.840, alt. c. 5 m) under Salix sp., 1 Oct. 2002, P. Boisen Hansen JV02-632, duplicate HJB10879. – FRANCE, Ain, Saint-Etienne-du-bois, Bourg (c. N46.26 W5.28; alt. c. 250 m) on wet ground with Sphagnum under Alnus glutinosa and Salix aurita, 19 Oct. 1964, G. Bruchet LY BR64-36, database record HJB1000067, this is the holotype of H. pusillum var. longisporum; Jura, Lac des Rouges Truites (c. N46.36252 E5.59531, alt. c. 940 m) in mixed woodland under Salix aurita, S. cinerea and S. caprea, 24 Sept. 1996, D. Aanen WBS 9644, database record HJB12515; Jura, Lac des Rouges Truites (c. N46.36252 E5.59531, alt. c. 940 m) in mixed woodland under Salix sp., 24 Sept. 1996, D. Aanen WBS 9648, database record HJB12518; Aude, Roquefeuil, Tourbiere du Pinet (N42.86421 E1.9775, alt. c. 880 m) in wet, boggy mixed woodland with Salix atrocinerea, 14 Oct. 2008, J.-P. Priou HJB12730; Ariege, Freychinede (N42.8031167 E1.4225500, alt. c. 1350 m) on wet boggy soil in woodland under Salix atrocinerea, 29 Sept. 2007, G. Corriol GC 07 09 29 03, duplicate HJB12107. – GERMANY, Bavaria, near Riedheim, Gunzberg, MTB7527/1 (c. N48.462 E10.189, alt. c. 456 m) under Betula sp., Salix sp., 9 Sept. 1994, M. Enderle M 0155166, database record HJB1000137, this is part of the holotype of H. vaccinum var. cephalotum; Berlin-Altglienicke, Grünauer Railway Junction, Flat Gk 1 (c. N52.4167 E13.55, alt. c. 35 m) on moist ground under Alnus sp. and Salix sp., 4 Oct. 1999, F. Gröger GLM GL42941, database record HJB10993. – NETHERLANDS, Drenthe, Vledder, Wagserveense (c. N52.84 E6.22, alt. c. 0 m) collected with Salix sp., 20 Sept. 1995, D. Aanen WBS 9507, database record HJB12810; Drenthe, Vledder, Wagserveense (c. N52.84 E6.22, alt. c. 0 m) collected with Salix sp., 20 Sept. 1995, D. Aanen WBS 9508, database record HJB12460; Drenthe, Vledder (c. N52.512692 E6.123788, alt. c. 5 m) collected with Salix sp., 20 Sept. 1995, D. Aanen WBS 9509, database record HJB12461; Zouweboezem, Meerkerk (c. N51.9539 E4.9858, alt. c. 0 m) under Salix aurita and S. cinerea, 20 Oct. 1996, D. Aanen WBS 9690, database record HJB12801. – WALES, Brecon, Illkyd Pools (c. N51.92107 W3.503536, alt. c. 320 m) on wet soil under Salix sp., 17 Oct. 2003, A. Andrews HJB9494.
Notes — Given the shape of its cheilocystidia, H. pusillum clearly belongs to H. subsect. Denudata. The species most likely corresponds to ICG8 of Aanen & Kuyper (1999). The strongly 2-coloured cap, somewhat reminiscent of H. mesophaeum but with much more slender stature and without cortina leaves very few possibilities. Other small Hebeloma spp. have not such strongly 2-coloured caps, nor are they so slender. We have no confirmed records of this taxon in arctic or alpine habitats. It can be readily distinguished from the other members of this subsection that grow in lowland areas based on the distinctly 2-coloured cap, the number of lamellae 20–40, the slender basidiomes with stipe width ≤ 3.5 mm and stipe Q normally > 10, the spore length, > 11 μm, the large average width of the cheilocystidium apex ≥ 8 μm, and with no regular apical thickening of the cheilocystidium. It has probably often been confused with H. helodes, which is also a small Hebeloma sp., but H. pusillum is confined to Salix, has a more fragile stature and usually a rather darker centre to the pileus and significantly longer spores, on average. Hebeloma luteicystidiatum also has long spores but the very distinct apical thickening of the cheilocystidium distinguishes this taxon from H. pusillum, which rarely has any apical thickening of the cheilocystidium. Hebeloma aurantioumbrinum, is rare in non-arctic/alpine environments but also has shorter spores on average. Hebeloma minus is also rare in non arctic/alpine environments but in any case this taxon has a smaller spore Q from that of H. pusillum. Hebeloma salicicola could be confused with H. pusillum but normally it has a more robust stature with a wider stipe, a smaller stipe Q and the spores of H. salicicola are on average more dextrinoid.
Hebeloma pusillum forms species clades in all of the loci tested, and receives bootstrap support in all single locus results apart from ITS.
Having studied the description and holotype material of H. vaccinum var. cephalotum we are convinced this was a mixed collection of H. salicicola, H. vaccinum and H. pusillum. We have extracted DNA several times and every sequence we have generated is of H. pusillum, similarly the material we have examined is H. pusillum. However, the macroscopic description given for this taxon very much more resembles H. salicicola. We have also examined the isotype of this material, but that is H. vaccinum. So we conclude that we should synonymise it with H. pusillum but point out that the macroscopic description does not really match H. pusillum. See also the discussion following the description of H. salicicola.
We have examined the description and holotype material of H. pusillum var. longisporum both morphologically and molecularly and have concluded that there are no grounds for separating this variety. This is in agreement with Gröger (1987). The molecular sequences we have generated are identical to H. pusillum and our measurements of the spore length show it to be in the middle of the range for this species; we have included Bruchet’s collection within the set of collections upon which our overall species description is based.
The original iconotype of Lange is an excellent representation of this taxon. The epitype selected by Vesterholt is also representative.
Hebeloma salicicola Beker, Vesterh. & U. Eberh., sp. nov. — MycoBank MB809912; Fig. 5h, 32, 33
Fig. 32.
Hebeloma salicicola (BR-MYCO 173977-56, holotype). a, b. Spores and spore ornamentation ×1 600 in Melzer’s reagent; c, d. spores and spore ornamentation ×1 600 in 5 % KOH; e, f. cheilocystidia ×1 000 in 5 % KOH; g. cheilocystidia ×500 in 5 % KOH; h. basidium ×1 000 in 5 % KOH; i. caulocystidia ×1 000 in 5 % KOH; j. cutis ×125 in 5 % KOH. — Scale bars: 10 μm, in j. 100 μm.
Fig. 33.
Hebeloma salicicola (BR-MYCO 173977-56, holotype). a. Basidia; b. spores; c. cheilocystidia. — Scale bars: 5 μm.
Etymology. Meaning lover of Salix, being exclusively mycorrhizal with Salicaceae.
Type. BELGIUM, De Panne, Westhoek, West Flanders (N51.08793 E2.57568, alt. c. 3 m) on sand in calcareous dune slack with Salix repens, 12 Oct. 2009, H. Beker, L. Davies holotype BR BR-MYCO 173977-56; isotype C C-F-90151, HJB13302.
Diagnosis — Hebeloma salicicola is a member of H. subsect. Denudata based on cheilocystidium shape, though the cystidia can be rather broad at the base. Macroscopically it is similar to H. vaccinum, but in contrast to H. vaccinum, mature specimens nearly always have a 2-coloured pileus with a darker centre. Microscopically H. salicicola differs from other members of its subsection by a combination of characters, i.e. its small stature, the low number of full length lamellae (< 50), the rather strong dextrinoidity (D2,D3) and ornamentation of the spores (O2,O3), which are on average > 11 μm.
Basidiomes usually in scattered groups, rarely solitary, sometimes gregarious. Pileus 10–48 mm diam, convex often umbonate, surface often viscid, tacky when moist but never hygrophanous; cuticle colour mature basidiomes are almost always 2-coloured with the centre from ochre to a rich red brown, dark brick or sepia and the margin from cream to clay pink or buff or Isabella; pileus margin usually straight but sometimes scalloped. Lamellae adnate or emarginated, sometimes almost free, usually quite distant with L = 30–49; maximum depth 2–9 mm; colour cream, alutaceous or brown when young, later umber to sepia following spore maturity; edge fimbriate, usually significantly paler than lamella surface; droplets sometimes visible on the lamella edge, but often absent; lamellules frequent. Stipe central, sometimes cylindrical but more often clavate or even appearing bulbous, particularly when collected in a sand dune with a bulb formed from sand, rarely tapering, becoming hollow with age, (5–)9–48(–56) × (1.5–)2–7 mm, and up to 11 mm at the base with stipe Q (5–95 %) ranging from 3.5–11.1, sometimes with slight discolouration towards the base of the stipe; surface dry, pruinose to floccose, especially towards the apex. Cortina not observed. Flesh thick, white to pale buff. Smell raphanoid, often with a cocoa smell, sometimes weak, sometimes absent. Taste usually bitter. Spore deposit brownish olive to umber. Exsiccata sometimes, but not always, dark coloured with blackening lamellae.
Spores amygdaloid or limoniform, sometimes fusoid, with small apiculus and rounded at the end opposite the apiculus, with some thinning of the spore wall, and often with a papilla, sometimes guttulate with one or more oily drops, sometimes weakly ornamented but usually more distinctly ornamented, sometimes with slightly loosening perispore in a few to many spores and distinctly to rather strongly dextrinoid with spores becoming orange brown or light brick in Melzer’s reagent (O2,O3; (P0)P1,P2; D2,D3); spore colour under the microscope from yellow through yellow brown to brown; spore size based on n = 54 spores of the holotype, 5–95 % percentile range 11.6–13.9 × 6.4–7.8 μm, with median 12.7 × 7.2 μm and av 12.7 × 7.1 μm with SD length 0.67 μm and width 0.46 μm, Q value 5–95 % percentile range 1.67–1.92, with median 1.78 and av 1.78 with SD 0.08; spore size based on 21 collections medians 11.2–13.3 × 6.0–7.6 μm and av 11.2–13.3 × 6.1–7.5 μm with SD length 0.50–1.24 μm and width 0.25–0.51 μm, Qav 1.64–2.00. Basidia cylindrical to clavate and 4-spored, 22–38 × 7.2–10.2 μm, with av 24–37 × 8.0–9.9 μm and basidium Qav in the range 3.0–4.1. Pleurocystidia not found. Cheilocystidia capitate-stipitate or clavate-stipitate, sometimes swollen towards the base (capitate-lageniform or clavate-lageniform), usually with some thickening of the apex, occasionally with septa and occasionally bifurcate; width of apex holotype 5–95 % percentile range 7.5–10.8 μm, with median 8.9 μm and av 8.9 μm with SD 1.01 μm; across 21 collections median 7.8–10.6 μm and av 7.9–10.7 μm; with n ≥ 20 selected cheilocystidia of 21 collections the 5–95 % percentile ranges are 37–77 × 6.1–15.5 × 3.0–5.7 × 2.5–8.9 μm while the averages are 46–63 × 7.9–10.7 × 3.8–5.0 × 3.6–5.9 while the av for the holotype was 54 × 8.9 × 4.3 × 4.9. The av cheilocystidia ratios for the 17 collections were: A/M = 1.83–2.63; A/B = 1.60–2.82; B/M = 0.96–1.27. Caulocystidia resemble cheilocystidia but with a more swollen base, up to 85 × 13 μm wide at the apex. Pileipellis is an ixocutis with an epicutis medium thick, 80–150 μm, embedded hyphae up to 10 μm broad, smooth or sometimes encrusted, hyaline or occasionally pigmented. Cutis orange brown and made up of cylindrical to isodiametric elements. Trama below subcutis contains cylindrical, ellipsoid, ovate and sausage-shaped elements up to 16 μm broad. Clamp connections present throughout the basidiome.
Habitat & Distribution — Hebeloma salicicola appears to be confined in its mycorrhizal association to Salix and Populus. It is common and often gregarious in calcareous dune slacks growing with Salix repens, where it appears to have two fruiting periods per year, in the spring and in the autumn. We also have one alpine record of H. salicicola with Salix herbacea and one arctic record where it was growing in association with Salix polaris. Other records (not from dunes or alpine or arctic) are from grassy or mossy, often sandy ground with Populus × alba or Salix sp. in scrub, gardens, grassland or woodland plantations, on both acid and calcareous soils.
Additional specimens examined. BELGIUM, prov. West Flanders, Westhoek (c. N51.04 E3.56, alt. c. 0 m) in dune on sandy, calcareous soil with Salix repens, 26 Apr. 2004, H. Beker HJB9911; prov. West Flanders, Westhoek (c. N51.04 E3.56, alt. c. 0 m) in dune on sandy, calcareous soil with Salix repens, 26 Apr. 2004, H. Beker HJB9913; prov. West Flanders, Westhoek (N51.08750 E2.5754833, alt. c. 0 m) in dune on sandy, calcareous soil with Salix repens, 6 Oct. 2004, H. Beker HJB10260; prov. West Flanders, Ter Yde (N51.1379 E2.69635, alt. c. 0 m) in dune on sandy, calcareous soil with Salix repens, 22 Oct. 2004, H. Beker HJB10422; prov. West Flanders, Westhoek (N51.08750 E2.5754833, alt. c. 0 m) in dune on sandy, calcareous soil with Salix repens, 22 Apr. 2005, H. Beker HJB10923; prov. Brabant-Flanders, Meise, Botanical Gardens (c. N50.92 E4.32, alt. c. 35 m) in rural gardens on mossy, calcareous soil with Salix sp., 1 May 2005, H. Beker HJB10926; prov. Antwerp, Linker oever (c. N51.216667 E4.416667, alt. c. 10 m) on calcareous soil with Salix sp., 16 Apr. 2000, J. Volders VJ00012, duplicate HJB11501; prov. West Flanders, Westhoek (N51.0875000 E2.5754833, alt. c. 0 m) on sandy soil in maritime dune under Salix repens, 6. Oct.10. 2004, H. Beker (HJB10254); prov. West Flanders, Westhoek (N51.0875000 E2.5754833, alt. c. -6 m) on sandy soil in maritime dune under Salix repens, 6 Oct. 2004, H. Beker HJB10261; prov. West Flanders, Ter Yde (N51.13790 E2.69635, alt. c. 8 m) on grassy soil in dune under Salix repens, 22 Oct. 2004, H. Beker HJB10427. – ESTONIA, Saaremaa, Kaarma Community, Mändjala, near Hotel Saaremaa (N58.21198 E22.31861, alt. c. 5 m) on sandy, calcareous soil with Pinus sylvestris and Salix sp., 19 Sept. 2008, J. Vauras C TURA JV08-278, duplicate HJB12323. – FINLAND, Uusimaa, Hanko, Tvärminne, Tvärminneby (c. N59.84 E23.2, alt. c. 10 m) on calcareous soil under Salix sp., 30 June 1998, J. Vauras C TURA JV13610, duplicate HJB10930. – FRANCE, Haute Savoie, Lac des assiettes (N45.39001 E6.78529, alt. c. 2478 m) on bare soil in mountain scrub under Salix herbacea, 23 Aug. 2009, L. Davies HJB13087. – NETHERLANDS, Groningen, Eemshaven (c. N53.448 E6.831, alt. c. 0 m) with Salix repens, 3 Oct. 1996, D. Aanen WBS 9670, database record HJB12473; Groningen, Eemshaven (c. N53.45 E6.83, alt. c. 0 m) with Salix repens, 3 Oct. 1996, D. Aanen WBS 9678, database record HJB12479; Lelystad, Oostvaardersplassen (Flevoland) (c. N52.45 E5.37, alt. c. 0 m) in dune with Salix repens, 11 Oct. 1995, D. Aanen WBS 9567, database record HJB12533. – POLAND, Mt Kamiensk (the outer damping ground of the Belchatow Lignite Mine) forest distr. 297 (by the pond) (N51.22136 E19.43898, alt. c. 340 m) conifer woodland plantation with Salix sp., 23 Sept. 2008, I. Kałucka, H. Beker HJB12677. – SPAIN, Madrid, Colmenarejo (c. N40.56 W4.01, alt. c. 890 m) in broadleaf plantation with Populus × alba, 17 Apr. 2001, F. Prieto HJB9072. – SVALBARD, Ossian (N78.9257000 E12.4542167, alt. c. 2 m) on grassy soil in maritime coastal scrub under Salix polaris, 17 Aug. 2007, H. Beker, M.L. Beker HJB12020. – WALES, Anglesey, Newborough (N53.1483800 W4.0425270, alt. c. 0 m) on sand in dune under Salix sp., 24 Sept. 2001, H. Beker HJB5117.
Notes — Given the shape of its cheilocystidia, H. salicicola clearly belongs to H. sect. Denudata and, although its cheilocystidia are perhaps more swollen in their lower half than those of many other species of H. subsect. Denudata, this taxon still falls within the subsection parameters. The species most likely corresponds to ICG14 of Aanen & Kuyper (1999). It appears restricted to Salix and Populus and can be common, even gregarious, in calcareous dunes where it is often growing alongside H. vaccinum with which, macroscopically, it can be confused particularly when young and still appearing almost unicoloured. In the key to this subsection we key it out in both subkeys. It can be distinguished from other arctic/alpine species of this subsection through the number of lamellae, < 60, and the spores more ornamented or more dextrinoid than H. aurantioumbrinum, H. alpinum, H. louiseae, H. minus, H. pallidolabiatum and H. perexiguum and usually without a very strong distinct papilla, unlike H. alpinum. It can be separated from other lowland species in this section through the number of full length lamellae 30–60, the spore length > 11 μm, the smallish stature and the dextrinoidity of the spores. In the past this species has probably been confused with H. vaccinum and perhaps H. pusillum in dunes and lowland areas and with H. minus in alpine and arctic habitats. Both ITS and RPB2 distinguish this species.
Originally, we referred to this taxon as ‘Hebeloma cephalotum’ as it matched closely with the description of H. vaccinum var. cephalotum Enderle & Vesterh. in Enderle (2004). However, as mentioned above, we have examined the holotype of this species (M M-0155166) and it appears to be a mixed collection. While some of the fragments we have examined may belong to H. salicicola, all fragments we have sequenced are of H. pusillum, which is certainly present, from a morphological perspective, in the material deposited. Similarly the microscopic morphological analysis we have carried out also corresponds to H. pusillum. Trying to resolve this, we also examined an isotype (C C43996) but this collection, based on both molecular and morphological study is H. vaccinum. We do believe that the material of H. vaccinum var. cephalotum studied by J. Vesterholt corresponded to the taxon we have called H. salicicola, however, given the mixed nature of both the holotype and the isotype of H. vaccinum var. cephalotum we conclude that it is safer to describe this taxon as new.
DISCUSSION
The evolutionary distance, on the whole small, between all members of H. subsect. Denudata and particularly between some members of the H. crustuliniforme complex, suggest that, in spite of the demonstrated differentiation between clades and morphotypes, their presumably short evolutionary history may not have been sufficient for species to be fully differentiated in all characters. The analyses presented here give an impression of how well the species characterized above are supported by different analyses. With a full dataset of five genes, all species, apart from H. alpinum, are monophyletic and all, apart from H. minus, receive bootstrap support. Missing data introduce uncertainty in the results, which could not be avoided, as we were aiming for the best possible geographical, morphological and ecological representation for every species on the basis of the available material. Confronted with probable coalescence and possible hybridization, leading to an increased probability of recombination, phylogenetic analysis methods are not the most appropriate analysis methods. Both coalescence-based species tree methods or population genetic methods might be more appropriate. These kinds of analyses are geared towards haploid and unlinked markers and call for knowledge of population parameters; requirements that the available data do not readily fulfil. While we are still in the process of exploring the applicability of these methods, we do not expect the results will be very different from the results presented here with regard to species delimitation.
The data presented above show that the DNA barcode (ITS) is, among the loci tested, perhaps the least well suited for discriminating between taxa of H. subsect. Denudata. A cut-off value of 3 % allows the separation of some H. pusillum and some H. eburneum sequences from the rest of the sequences, but the overwhelming majority of ITS sequences are more similar than 97 % and defied species identification with a 3 % cut-off. It is obvious from Table 1 that a blast search based identification based on any cut-off values of ITS similarity is bound to fail, if requiring all matches below a certain cut-off value to be assigned to the same species. In spite of this, complete ITS sequences can usually be correctly assigned to a species, because normally the great majority of very good matches belong to a single taxon and the great majority of sequences belonging to other species have noticeably worse scores. Furthermore, once the ITS data will be entered into the species hypotheses clustering of UNITE (Kõljalg et al. 2013), we will be able to annotate the sequences in a way, so that the users of this database will be aware of the identification caveats within this group of species.
The ML results suggest that the RPB2 is the most powerful single locus for recognizing species, but H. aanenii, H. alpinum and H. geminatum do not form monophyla. Obviously, this result may be biased due to the smaller number of sequences in the RPB2 dataset as opposed to some others, namely ITS.
In our analyses, we found eight new species within H. subsect. Denudata, for which old names were not available. They are described above as Hebeloma aanenii, H. aurantioumbrinum, H. geminatum, H. louiseae, H. luteicystidiatum, H. pallidolabiatum, H. perexiguum and H. salicicola, alongside descriptions of our concepts of known species (H. alpinum, H. eburneum, H. helodes, H. lutense, H. minus and H. pusillum). For all of these taxa, type material was studied. For the concept adopted for H. crustuliniforme (H. crustuliniforme (Bull.) Quél. emend. Vesterh., U. Eberh. & Beker) see Vesterholt et al. (2014). Molecular results suggest that the H. crustuliniforme complex includes nine taxa in Europe (H. aanenii, H. alpinum, H. crustuliniforme, H. eburneum, H. geminatum, H. louiseae, H. minus, H. pallidolabiatum and H. salicicola), of which H. alpinum is the least distinctive in molecular terms (followed by H. minus and H. geminatum), and H. aanenii and H. geminatum in morphological terms. Above, in the species comments of the taxonomy part of the paper, we discuss the lines of evidence leading to the acceptance of the respective species and how the species can be recognized.
The species classification adopted here is superficially in stark contrast to the taxonomical conclusions of Aanen & Kuyper (2004) who, also with regard to the determination of species by morphology alone, included H. aanenii, H. alpinum, H. crustuliniforme, H eburneum, H. geminatum and H. salicicola as adopted here in H. crustuliniforme. Hebeloma helodes, H. luteicystidatum, H. lutense, H. minus, H. pusillum and some other taxa outside of H. subsect. Denudata as defined here were merged in H. helodes. The latter species (H. helodes sensu Aanen & Kuyper 2004) was entirely based on morphology, not on intercompatibility test results, adopting a wide morphological concept. This concept is also not supported by the results of molecular analyses presented here, clearly showing a number of well-supported taxon clades, matching with morphological and ICG data.
Another important reason for the different morphological conclusions between this work and that of Aanen & Kuyper (2004) is that they used cheilocystidia length and width where here width ratios between different parts of the cheilocystidia were used to better capture cheilocystidia shapes. This does also explain why their concept of H. helodes does not fit into our concept of H. subsect. Denudata, which is strongly based on cheilocystidium shape. In addition, Aanen & Kuyper (2004) appear to have used size classes for spore measure means rather than the means of the measures themselves.
In the case of H. crustuliniforme sensu Aanen & Kuyper the species delimitation of Aanen & Kuyper (2004) was also related to intercompatibility between some members of different ICGs and the observation of a strain of ambiguous ICG membership. Their (Aanen & Kuyper 2004) reluctance to split H. crustuliniforme and H. helodes sensu auct. was further fed by molecular results based on ITS and IGS (intergenic spacer of the nuclear ribosomal RNA genes) data that – analoguous to some results presented here – show that neither all ICGs nor all morpho-species readily form monophyletic clades in gene trees.
However, taking a different view on the intercompatibility test results, one could also argue as we are doing here: If indeed the great majority of basidiomes can unambiguously be assigned to a single ICG (Aanen & Kuyper 1999), it stands to reason that at least some ICGs do represent biological species, which are then likely to have evolved a distinct morphology. We did not use ICG membership as a criterion for species delimitation. In spite of occasional intercompatibility between different ICGs (Aanen & Kuyper 1999) in the core of the H. crustuliniforme complex (H. aanenii, H. alpinum, H. eburneum, H. geminatum), there is good correspondence between ICG data and the species limits used here, in that all collections belonging to the same ICG are assigned to the same species, none of the species includes more than one ICG. (The special case of collection WBS 9605, database record HJB12498, compatible ICG3 and ICG4 and included in the same clade as other ICG4 members, is discussed below in more detail.) Eleven of the species recognized by us here appear to correspond to ICGs of Aanen & Kuyper (1999) and should accordingly be biological species. Apart from H. aanenii and H. geminatum all of these species can also be unambiguously separated based on morphology. This could be taken as an indication that hybridization, i.e. crosses between different ICGs, does not play a major role in nature. A contributing factor could well be that the two species corresponding to the most promiscuous ICGs according to Aanen & Kuyper’s (1999) results, H. alpinum and H. eburneum, though widespread and common, are separated by their respective ecologies, in that the first is restricted to arctic and alpine areas where the latter does not occur. This is rather suggestive of speciation by isolation in progress, with residual intercompatibility in some genotypes (Aaenen et al. 2000). The intercompatibility results (Aanen & Kuyper 1999) indicated possible, but less clear, intercompatibility of representatives of H. geminatum and H. aanenii and H. alpinum and H. aanenii. Both hybridisation and coalescence might be responsible for the lack of monophyly in some taxa and species clades in the shape of badly supported short-stemmed monophyla. The example of H. alpinum WBS 9605 (ICG 3/4) shows that on the basis of the molecular and morphological data we obtained, genotypes with exceptional biological intercompatibility cannot be detected. We do not know whether WBS 9605 could have produced viable offspring with a member of ICG3 and how the progeny might look. However, if viable offspring resulted, one would expect contradictions between morphology and genotype as in HJB11051, which is morphologically H. alpinum (ICG4) and genetically, based on five markers, H. geminatum (ICG1). Further, if HJB11051 was the direct product of hybridization, one would expect phased nuclear markers to belong to different taxon clades. This is not the case (results not shown). It is the only collection of H. alpinum we have from Iceland, and, given the relative vicinity of Iceland with Greenland, and given that we know that H. alpinum as delimited in Europe exists in Greenland, this might be an indication that the species delimitation between H. geminatum and H. alpinum as it works in Europe, might not do so with American material. In all of the 273 collections we examined molecularly, HJB11051 was the only one where a clear contradiction between genotype and morphology occurred.
It is tempting to see a key property of the H. crustuliniforme complex, actually of all species of H. subsect. Denudata, in their association to Salix ssp. While all the taxa of this subsection have been recorded with Salix, some of the taxa seem to only associate with this host genus (or with Salicaceae), see Table 3. Though the results reported by Tedersoo et al. (2013) implicitly caution against assuming that ectomycorrhizal taxa associating with one species of Salix would automatically form associations easily with other species of Salix, association with Salix could be considered an advantage to thrive during the climatic changes that took place in the northern hemisphere in the Pleistocene, and also to acquiring a Palearctic distribution.
Hebeloma aurantioumbrinum, one of the less common species in Europe, has been found in North America. Hebeloma alpinum is known from Greenland. It is likely that at least the cold-adapted taxa like H. alpinum, H. minus and possibly also H. aanenii, H. geminatum and H. salicicola are circumpolar species. Sequence data from the US and Canada is publicly available, but as the results presented here show, ITS diversity is not a reliable predictor for species diversity in this group of fungi. Reviewing a number of environmental sequencing studies of ectomycorrhizal fungi from arctic northern hemisphere environments, Timling & Taylor (2012) reported that Hebeloma spp. are among the fungal lineages that were recovered in all studies with at least moderate frequencies. Based on a 97 % cut-off value they concluded, that almost 3/4 of the arctic phylotypes of ectomycorrhizal agarics can also be found outside the arctic. The results presented here for H. subsect. Denudata cast serious doubt on the appropriateness of such a statement, firstly with view to methodology – a 97 % cut-off value is far too crude to capture spatial genetic structure in the genus Hebeloma; and secondly with regard to conclusions. There is a species overlap between arctic and alpine places, but the species overlap between arctic and temperate or boreal areas is small, if frequencies are also considered. Taxa that allegedly occur in both biomes (i.e., H. aanenii, H. aurantioumbrinum, H. geminatum, H. minus, H. salicicola), have a strong preference either for the arctic/alpine areas or lesser latitudes and altitudes, and must, according to our database, be considered rare in their less favoured habitats. It is of course possible that the taxa that were so far only collected in Svalbard (H. louiseae, H. pallidolabiatum and H. perexiguum) are North American taxa and perhaps not restricted to arctic or alpine habitats. Given the low level of interspecific variation in H. subsect. Denudata, and the probability of intercompatibility of at least some members of some species to mate with members of other taxa, it appears unlikely, that putative North American taxa have persisted unchanged in Svalbard since the islands’ isolation, given the geological and vegetation history of the islands (summarized by Geml et al. 2012). Long-distance dispersal may or may not be frequent from boreal or temperate regions to northern arctic environments (Geml et al. 2012), but one could argue that not dispersal but climatic selection does play a key role in the arctic Hebeloma flora.
Dispersal in the other direction, from the arctic to boreal and temperate zones, might be a lot more successful, when looking at the example of H. alpinum. This strictly arctic/alpine taxon is a rather common species (around 80 records on the database) and associates with Salix or Dryas. It is most difficult to distinguish molecularly and could be confused with H. aanenii, H. eburneum and H. geminatum, which have wide host spectra and do not (typically) occur under arctic/alpine conditions. The situation is characterised by shared alleles and there is little evidence of shared divergence within H. alpinum compared to its temperate sistertaxa which all show specific differentiation in a subset of the markers used. Incidentally, H. alpinum very likely corresponds to the most promiscuous ICG (Aanen & Kuyper 1999).
Compatibility is a driver of speciation (Aanen et al. 2000). According to the results of Aanen and co-workers (Aanen & Kuyper 1999, Aanen et al. 2000) it is a quantitative rather than purely qualitative trait. This is true for pairings within the same ICG as well as between members of different ICGs. Loss of compatibility may be precluded or followed by differentiation in morphology and genetic divergence in parts of the genome not directly involved in intercompatibility. While speciation and divergence are still in progress, every individual is likely to be a mosaic of genes and traits with different evolutionary histories. It appears likely that in the ancestral population of H. subsect. Denudata and in particular the ancestor of the H. crustuliniforme complex (with the possible exception of H. crustuliniforme itself) loss of compatibility and divergence in other characters did not always follow the same pattern. Possibly H. alpinum has maintained most of the traits of this ancesteral population, retaining more ancestral variation, staying faithful to arctic/alpine habitats and associating with Dryas and Salix, but not with conifers or other broadleaves (non-arctic/alpine Salix) and preserving the highest level of intercompatibility (Aanen & Kuyper 1999). Based on the data we have it is impossible to unambiguously trace the evolutionary history of the species of H. subsect. Denudata. The climatic oscillations during the Quaternary might even have arrested the divergence of H. alpinum and intercompatible taxa, potentially bringing intercompatible members of different taxa in touch that normally would live in separate habitats or geographically distant areas, i.e. Europe and America. On a side note, it is remarkable that the closest relative of H. sect. Denudata, H. mediorufum (Rees et al. 2013) associates with Nothofagus and seems restricted to New Zealand, where Salix is not endogeneous.
On an evolutionary timescale, species are transient. In cases like the H. crustuliniforme complex and related taxa it is to an extent subjective whether to consider divergence and non-compatibility as sufficiently advanced to separate groups of organisms into species. One might argue that it does not make sense to recognize or even describe species that are likely to be unidentifiable without huge effort and using identification tools that may not be widely used today. It may be argued that a consequence is that misnamed sequences and collections might crowd databases and be misleading rather than elucidating. Indeed, it is also possible that what we describe here as species may turn out to be somewhat differentiated sub-populations of to-date unstudied larger entities that are better suited to represent species. However, we believe that the dataset that forms the basis of this study is sufficiently extensive and sufficiently consistent to recognize entities, here described as species, that have diverged in the context of the climatic, geological and biological history of Europe.
Acknowledgments
We are very much obliged to G. Walther for providing microscopic drawings. The help of A. Bogaerts from the herbarium in Meise (BR) and of H. Knudsen (C) for handling loans and managing deposits is greatly appreciated. Great thanks to Th.W. Kuyper for kindly making available to us material used by D.K. Aanen from WBS, now L. For help in translating various papers and descriptions we thank J. Kleine and M. Noordeloos. The authors would like to thank the herbaria in C, E, G, GLM, IB, K, L, LOD, LY, M, MKNH, MPU, O, OULU, P, PDD and TURA for the loan of collections for study and sequencing, BR for supplying material for morphological and for molecular analyses. Furthermore, we very much appreciated the help of A. Andrews, P. Antibus, V. Antonin, P. Ardron, M.L. Beker, E. Bendiksen, P. Boisen Hansen, T. Borgen, A. Brand, P. Cavanagh, G. Corriol, C. Cripps, L. Davies, D. Deschutyer, E. Emmett, M. Enderle, D. Ghyselinck, M. Ghyselinck, A. de Haan, H. Hallgrímsson, C. Hobart, E. Horak, S. Huhtinen, I. Kałucka, D. Karasinski, S. Kelly, T. Kirk, H. Knudsen, T. Læssøe, P. Larsen, C. Lecuru, M. Lenne, P. Leonard, P-A. Moreau, E. Ohenoja, I. Olariaga Ibarguren, J. Petersen, F. Prieto, J-P. Priou, M. Rotheroe, P. Roux, J. Sandmo, D. Schafer, U. Soderholm, K. Soop, M. Storey, A. Szczepkouski, A. Taylor, J. Vauras, J. Volders and any other collectors we may have forgotten by accident, for supplying us with interesting and exciting Hebeloma collections. We thank P. Derboven for the permission to use his photograph. Numerous people have helped in the lab to generate Hebeloma sequence data that were used directly or indirectly in this study. We would like to thank K. Dukik (CBS-KNAW Fungal Biodiversity Centre Utrecht), U. Fürst, S. Garnica and J. Schade (Universität Tübingen), and R. Gadjieva, M. Jonsson, C. Lundström, J. Petterson and D. Öncü (SLU Uppsala) as well as the Uppsala University Genome Center and the Hubrecht Institute sequence facility.
REFERENCES
- Aanen DK.1999. Species and speciation in the Hebeloma crustuliniforme complex. PhD thesis, Wageningen Universiteit, Netherlands. [Google Scholar]
- Aanen DK, Kuyper TW.1999. Intercompatibility tests in the Hebeloma crustuliniforme complex in northwestern Europe. Mycologia 91: 783–795. [Google Scholar]
- Aanen DK, Kuyper TW.2004. A comparison of a biological and phenetic species concept in the Hebeloma crustuliniforme complex within a phylogenetic framework. Persoonia 18: 285–316. [Google Scholar]
- Aanen DK, Kuyper TW, Boekhout T, et al. 2000. Phylogenetic relationships in the genus Hebeloma based on ITS1 and 2 sequences, with special emphasis on the Hebeloma crustuliniforme complex. Mycologia 92: 269–281. [Google Scholar]
- Aanen DK, Kuyper TW, Hoekstra RF. 2001. A widely distributed ITS polymorphism within a biological species of the ectomycorrhizal fungus Hebeloma velutipes. Mycological Research 105: 284–290. [Google Scholar]
- Boekhout T. 1982. De sekties Hebeloma (Fr.) Saccardo en Anthracophila Boekhout nom. prov. van het geslacht Hebeloma (Fr.) Kummer in Nederland en aangrenzende gebieden. PhD thesis, Leiden University, Netherlands. [Google Scholar]
- Bohus G. 1972. Hebeloma studies I. Annales Historico-Naturales Musei Nationalis Hungarici 64: 71–78. [Google Scholar]
- Borchsenius F. 2009. FastGap version 1.2. Department of Biosciences, Aarhus University; http://www.aubot.dk/FastGap_home.htm. [Google Scholar]
- Boyle H, Zimdars B, Renker C, et al. 2006. A molecular phylogeny of Hebeloma species from Europe. Mycological Research 110: 369–380. [DOI] [PubMed] [Google Scholar]
- Eberhardt U, Beker HJ.2010. Hebeloma vesterholtii, a new species in section Theobromina. Mycological Progress 9: 215–223. [Google Scholar]
- Eberhardt U, Beker HJ, Vesterholt J, et al. 2013. European species of Hebeloma section Theobromina. Fungal Diversity 58: 103–126. [Google Scholar]
- Eberhardt U, Beker HJ, Vila J, et al. 2009. Hebeloma species associated with Cistus. Mycological Research 113: 153–162. [DOI] [PubMed] [Google Scholar]
- Enderle M. 2004. Die Pilzflora des Ulmer Raumes. Verein für Naturwissenschaft und Mathematik in Ulm e.V., Ulm, Germany. [Google Scholar]
- Geml J, Timling I, Robinson CH, et al. 2012. An arctic community of symbiotic fungi assembled by long-distance dispersers: phylogenetic diversity of ectomycorrhizal basidiomycetes in Svalbard based on soil and sporocarp DNA. Journal of Biogeography 39: 74–88. [Google Scholar]
- Grilli E. 2007. Type studies in Hebeloma unravelling a taxonomic-nomenclatural tangle: What is Hebeloma leucosarx? Micologia e Vegetazione Mediterranea 22: 133–176. [Google Scholar]
- Gröger F. 1987. Der Formenkreis des winzigen Fälblings, Hebeloma pusillum. Mykologisches Mitteilungsblatt 30: 37–48. [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT Multiple Sequence Alignment Software version 7: Improvements in performance and usability. Molecular Biology and Evolution. doi:10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauff F, Lutzoni F. 2002. Phylogeny of the Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits. Molecular Phylogenetics and Evolution 25: 138–156. [DOI] [PubMed] [Google Scholar]
- Kõljalg U, Nilsson RH, Abarenkov K, et al. 2013. Towards a unified paradigm for sequence-based identification of fungi. Molecular Ecology 22: 5271–5277. [DOI] [PubMed] [Google Scholar]
- Lanfear R, Calcott B, Ho SYW, et al. 2012. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29: 1695–1701. [DOI] [PubMed] [Google Scholar]
- Lange JE. 1938. Studies in the Agarics of Denmark. Part XII. Hebeloma, Naucoria, Tubaria, Galera, Bolbitius, Pluteolus, Crepidotus, Pseudopaxillus, Paxillus. Dansk botanisk Arkiv 9: 1–104. [Google Scholar]
- Maddison WP, Maddison DR. 2011. Mesquite: A modular system for evolutionary analysis version 2.75, http://mesquiteproject.org. [Google Scholar]
- Malençon G, Bertault R. 1970. Flore des champignons supérieurs du Maroc I. Travaux de l’Institut scientifique chérifien et de la Faculté des sciences. Series Botanique et biologie végétale 32: 440–465. [Google Scholar]
- Marmeisse R, Gryta H, Jargeat P, et al. 1999. Hebeloma. In: Cairney JWG, Chambers SM. (eds), Ectomycorrhizal fungi: Key genera in profile: 89–127. Springer, Berlin, Germany. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, 2010: 1–8. [Google Scholar]
- Moser M.1970. Beiträge zur Kenntnis der Gattung Hebeloma. Zeitschrift für Pilzkunde 36: 61–75. [Google Scholar]
- Moser M.1985. Beiträge zur Kenntnis der Gattung Hebeloma II. Sydowia. Annales Mycologici editi in Notitiam Scientiae Mycologicae Universalis, Series II, 38: 171–177. [Google Scholar]
- Queiroz K de. 2007. Species concepts and species delimitation. Systematic Biology 56: 879–886. [DOI] [PubMed] [Google Scholar]
- R Core Team 2014. R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org/. [Google Scholar]
- Rambaut A. 2012. FigTree. Tree figure drawing tool version 1.4.0, Institute of Evolutionary Biology, University of Edinburgh: http://tree.bio.ed.ac.uk/. [Google Scholar]
- Rees BJ, Midgley DJ, Marchant A, et al. 2013. Morphological and molecular data for Australian Hebeloma species do not support the generic status of Anamika. Mycologia 105: 1043–1058. [DOI] [PubMed] [Google Scholar]
- Simmons MP, Ochoterena H. 2000. Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology 49: 369–381. [PubMed] [Google Scholar]
- Stamatakis A.2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web serversx. Systematic Biology 57: 758–771. [DOI] [PubMed] [Google Scholar]
- Sulzbacher MA, Grebenc T, Jacques RJS, et al. 2013. Ectomycorrhizal fungi from southern Brazil – a literature-based review, their origin and potential hosts. Mycosphere 4: 61–95. [Google Scholar]
- Tedersoo L, May TW, Smith ME. 2010. Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 20: 217–263. [DOI] [PubMed] [Google Scholar]
- Tedersoo L, Mett M, Ishida TA, et al. 2013. Phylogenetic relationships among host plants explain differences in fungal species richness and community composition in ectomycorrhizal symbiosis. New Phytologist 199: 822–831. [DOI] [PubMed] [Google Scholar]
- Timling I, Taylor DL. 2012. Peeking through a frosty window: molecular insights into the ecology of arctic soil fungi. Fungal Ecology 5: 419–429. [Google Scholar]
- Vaidya G, Lohman DJ, Meier R. 2011. SequenceMatrix: concatenation software for the fast assembly of multigene datasets with character set and codon information. Cladistics 27: 171–180. [DOI] [PubMed] [Google Scholar]
- Vesterholt J.2005. The genus Hebeloma, vol. 3. Fungi of Northern Europe. Svampetryk, Tilst, Denmark [Google Scholar]
- Vesterholt J, Eberhardt U, Beker HJ. 2014. Epitypification of Hebeloma crustuliniforme. Mycological Progress 13: 553–562. [Google Scholar]
- Vu VQ. 2011. ggbiplot: A ggplot2 based biplot. R package version 0.55. http://github.com/vqv/ggbiplot. [Google Scholar]