Fig. 2.
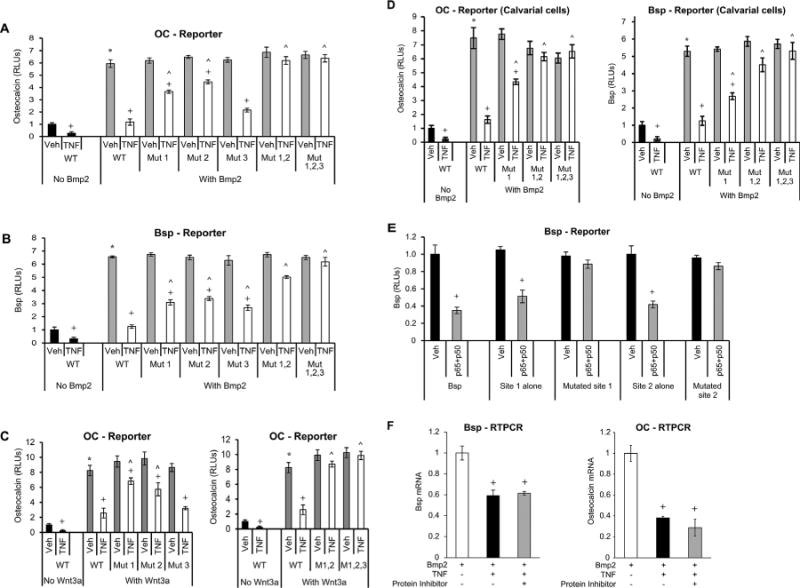
Mutation of NF-κB binding sites in osteocalcin or Bsp promoters rescues the negative effect of TNFα, indicating a direct effect of NF-κB. NF-κB binding sites were mutated as indicated (Mut1; Mut2; Mut3; Mut1,2; Mut1,2,3). Cells were transfected with wild-type and mutant constructs. After a 1-hour incubation with TNFα, cells were stimulated with Bmp2 or Wnt3a as indicated. (A–C) OC or Bsp promoter activity was analyzed by luciferase reporter construct. Luciferase activity was normalized by renilla control and expressed as relative luciferase units (RLUs). (D) Primary mouse osteoblastic cells were transfected with osteocalcin or Bsp reporter construct with or without mutated NF-κB binding sites. Transcriptional activity was measured using a luciferase assay and expressed as relative luciferase units (RLUs). (E) Cells were transfected with wild-type or mutated Bsp luciferase constructs containing all the NF-κB putative sites. Using subcloning, Bsp mini genes were prepared from wild-type and mutated promoter regions as described in Materials and Methods. NF-κB was activated by cotransfecting cells with p65- and p50-overexpressing plasmids. (F) Cells were stimulated with Bmp2 for 48 hours and then incubated with TNFα for 6 hours without or with cycloheximide. Bsp or OC mRNA levels were analyzed by RT-PCR. *Significantly different from Bmp2-stimulated control; +significant inhibition with TNFα; ˄significantly different between cells transfected with WT (wild-type construct) versus matched control (p < 0.05).
