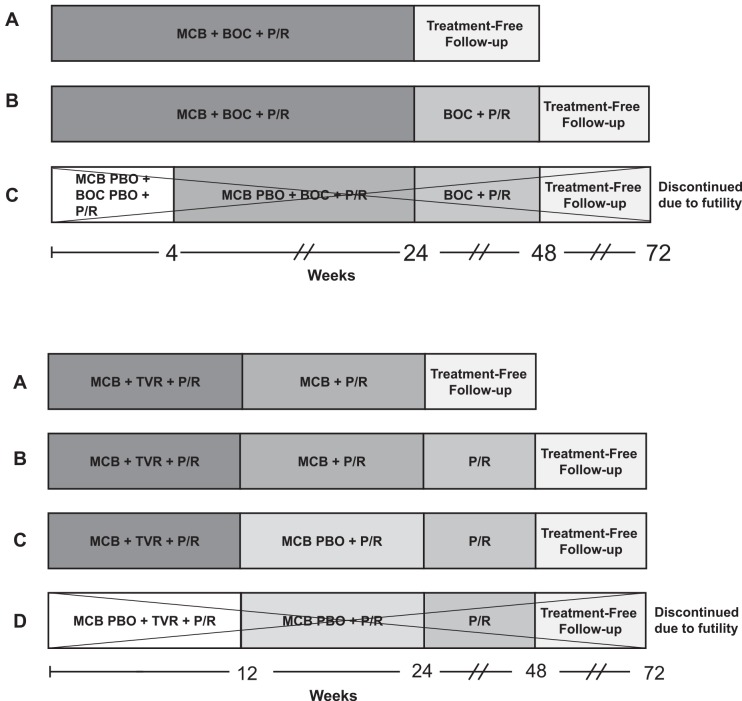
Fig 3. Study designs of DYNAMO 1 (a) and DYNAMO 2 (b).
BOC, boceprevir 800 mg TID (at recommended intervals of 7–9 hours); MCB, mericitabine 1000 mg BID; P/R, peginterferon alfa-2a 180 μg once/week + ribavirin 1000 mg/day (<75 kg) or 1200 mg/day (≥75 kg); PBO, placebo; TVR, telaprevir 750 mg TID (at recommended intervals of 7–9 hours). Control Arms C in DYNAMO 1 and D in DYNAMO 2 were closed due to futility while the studies were ongoing (27 July 2012 in DYNAMO 1 and 10 July 2012 in DYNAMO 2), after which patients in these groups were given the option to receive mericitabine for a maximum duration of 24 weeks. MCB was added to the regimen for 5 patients in Arm C in DYNAMO 1 and to the regimen for 9 patients in Arm D in DYNAMO 2 at various time points and for various durations.