Abstract
We aim to investigate the variation of CD44v6 expression in the normal-adenoma-primary carcinoma-liver metastasis sequence and its prognostic impact on colorectal carcinomas. The difference in CD44v6 expression between the tumor center and invasive front was also assessed. Immunohistochemistry was performed for CD44v6 on two cohorts. The first was tissue microarrays including 402 primary CRCs sampled from the tumor center and the invasive margin. The second was whole-tissue sections, consisting of 217 adenomas, 72 primary carcinomas, and the corresponding metastatic carcinomas. In the first cohort, we found that CD44v6 down-regulation was inclined to lymph node metastasis and perineural invasion, and had an unfavorable prognosis compared with CD44v6 up-regulation. In the second cohort, CD44v6 expression was predominant in adenoma over primary carcinoma and liver metastasis in multiple steps (normal < adenoma > primary carcinoma and liver metastasis). In addition, our analysis showed that CD44v6 expression was decreased at the invasion front of the CRC compared with the center of the tumor. In conclusion, the maximal expression of CD44v6 in adenoma plays a crucial role in colorectal carcinogenesis, while loss of CD44v6 expression on the cell surface of the tumor edge enhances the progression of metastasis. CD44v6 down-regulation is an independent prognostic factor for strikingly worse disease-specific survival.
Keywords: CD44v6, adenoma, colorectal carcinoma, metastasis, immunohistochemistry
Introduction
CD44v6, an alternative splicing of the CD44 gene, is a member of the CD44 family of glycoproteins [1,2]. Overexpression of CD44v6 is well-documented in colorectal carcinomas (CRCs) by immunohistochemistry, but its prognostic role in CRC remains controversial. Many investigations proved increased CD44v6 expression was a risk factor on survival time in patients [3-9], while some showed opposite results [10-14]. Moreover, no relationship between the expression and prognosis was also observed in several studies [15-22]. It is accepted that adenoma is an essential part of multiple steps of CRC, and CD44v6 expression in adenoma has been inconsistently reported [15,23-26]. Hence, the function of CD44v6 expression in CRC needs to be evaluated.
In our study, we evaluated the expression of CD44v6 by immunohistochemistry analysis in order to establish its prognostic effect on 402 primary CRCs, define the expression pattern in the adenoma-primary carcinoma-liver metastasis sequence in 217 adenomas, 72 primary carcinomas, and matched liver metastasis, and investigate the expression difference between the tumor center and invasive front in 72 primary and corresponding metastatic carcinomas.
Materials and methods
Clinical data
Two cohorts of CRC tissues were constructed in this study. The first cohort was tissue microarrays (TMAs) including 402 primary colorectal tumors from the tumor center and the invasive margin. These tissues were from patients with CRC who underwent intentionally curative surgical resection at the People’s No. 1 Hospital of Xiaoshan, Hangzhou, between 1991 and 2006. We collected the tissue blocks from the Department of Pathology of that hospital. The median age at diagnosis was 65 years (range, 24-91); other clinicopathological features are listed in Table 1. Tumor budding was included in addition to the classical pathological features. This refers to microscopic clusters of undifferentiated cancer cells (≤ 5) ahead of the invasive margin of the cancer. Numbers from 0 to 4 were considered negative; ≥ 5 was considered positive. Tumor budding tends to worsen the prognosis of CRC patients in our previous work [27] and other reports [28]. None of the patients had received chemotherapy or radiotherapy before surgery. The median time of follow-up was 33 months (range 1-186). The second cohort was whole-tissue sections; 217 adenomas, 72 primary colorectal tumors, and matched liver metastases were included. These tissues were collected from the Department of Pathology of Sir Run Run Shaw Hospital, Hangzhou. The 217 adenomas were subdivided into 105 low- and 112 high-grade dysplasias using standard criteria. Seventy-two patients had matched primary and metastatic tumors. This group consisted of 58 synchronous and 14 metachronous metastases. Patients’ median age at the time of diagnosis was 58 years (range, 28-78). This study was approved by the Ethics Committee of Biomedicine, Zhejiang University.
Table 1.
Clinicopathological characteristics of the patients
| Clinicopathological Feature | n (%) |
|---|---|
| Age | |
| < 60 | 147 (36.6) |
| ≥ 60 | 255 (63.4) |
| Sex | |
| Male | 219 (54.5) |
| Female | 183 (45.5) |
| Location | |
| Colon | 209 (52) |
| Rectum | 193 (48) |
| Histotype | |
| Tubular | 336 (83.6) |
| Mucinous, signet ring and undifferentiated | 66 (16.4) |
| pT stage | |
| pT1-2 | 80 (19.9) |
| pT3-4 | 322 (80.1) |
| pN stage | |
| pN0 | 215 (53.5) |
| pN1-2 | 187 (46.5) |
| pM stage | |
| pM0 | 364 (90.5) |
| pM1 | 38 (9.5) |
| Vascular invasion | |
| Negative | 344 (85.6) |
| Positive | 58 (14.4) |
| Perineural invasion | |
| Negative | 267 (66.4) |
| Positive | 135 (33.6) |
| Tumor budding | |
| Negative | 316 (78.6) |
| Positive | 86 (21.4) |
Immunohistochemistry
After deparaffinization and rehydration, paraffin-embedded tissue sections (4 µm thick) were treated with heat-induced antigen retrieval buffer (pH 6.0 citrate buffer) under high pressure for 2 min. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide in absolute methanol at room temperature for 15 min. Serum blocking was performed using 10% normal goat serum (Liankebio, Hangzhou, Zhejiang, China) for 30 min. Then the tissue sections were incubated with primary antibodies to CD44v6 (clone VFF-18, mouse monoclonal, 1:1000 dilution, Abcam, Cambridge, UK) and E-cadherin (clone NCH-38, mouse monoclonal, 1:100 dilution, Dako Cytomation, Carpinteria, CA, USA) overnight at 4°C. The following day, the slides were washed three times in 0.1 M phosphate-buffered saline (PBS; pH 7.4). Then the signal was amplified using a Polink-2 Plus IHC Detection System using the PV-9001 Reagents kit (GBI, Bothell, WA, USA) two-step method following the manufacturer’s instructions. Diaminobenzidine (Zhongshan Goldenbridge Biotechnology, Beijing, China) was used as the chromogenic substrate, and counterstaining was performed with hematoxylin. For negative controls, the primary antibodies were replaced with PBS as a negative. Immunohistochemical scores for each section were scored by two experienced pathologists independently in a double-blinded manner. For CD44v6 and E-cadherin, only membranous staining was evaluated. The staining intensity (SI) was categorized into 4 groups (0-3): none (0), weak (1), moderate (2), and strong staining (3). The proportion of positively-stained cells (PP) was also semi-quantitatively estimated and classified as follows: 0, ≤ 5% staining; 1, 6-25%; 2, 26-50%; 3, 51-75%; and 4, ≥ 75%. SI and PP were multiplied to give an immunohistochemical score (IHS = SI × PP). The expression of CD44v6 and E-cadherin was semi-quantitatively assessed as IHS. Therefore, the combined IHS score ranged from 0 to 12. Based on receiver-operating characteristic curve analysis, cases were categorized into two groups: IHS = 0 (negative) and HIS ≥ 1 (positive).
Statistical analysis
Pearson’s χ2 or a 2-independent non-parametric test (Mann-Whitney) was used to evaluate the association between CD44v6 expression and clinicopathological parameters. Disease-specific survival (DSS) was defined as the time from the date of surgery to the date of death from CRC or last follow-up. Kaplan-Meier method was used to draw the survival curves. The differences between curves were tested by the log-rank test. Multivariate survival analyses were performed using the Cox proportional hazard regression model. The Wilcoxon signed ranks test was used to analyze the paired data among normal mucosa, primary carcinoma, and liver metastasis. The Mann-Whitney non-parametric test was used for 2-independent samples. Significance levels for all tests were set to α = 5% in a two-tailed manner. Statistical procedures were done using IBM SPSS 20.0 statistical software (SPSS Inc. Chicago, IL, USA).
Results
Expression of CD44v6 in adenoma, primary carcinoma, and liver metastasis patients
Immunohistochemical analysis showed that most CD44v6-positive cells were located in the lower half of normal crypts, while the staining of colon carcinomas for CD44v6 was diffuse and strong in TMAs and whole tissues. In the first TMA cohort, the percentage of positive CD44v6 expression was 22.9% in normal tissue and markedly higher in CRCs (72.6%, P < 0.01).
The second cohort was used to identify the variation of CD44v6 expression in the normal-adenoma-primary carcinoma-liver metastasis sequence. CD44v6 expression in adenoma was maximal, while it was down-regulated in primary carcinoma and liver metastasis. There was no difference between primary carcinoma and liver metastasis. It must be pointed that this reduction was only relative, and the overall CD44v6 expression in primary carcinoma and liver metastasis specimens was still higher compared with normal mucosa (normal < adenoma > primary carcinoma and liver metastasis) (Table 2; Figure 1). No difference was found between adenomas with low- and high-grade dysplasia (Mann-Whitney non-parametric test, P = 0.936).
Table 2.
CD44v6 expression during the colorectal adenoma-carcinoma-metastasis sequence in the second cohort
| group 1 | group 2 | P |
|---|---|---|
| N | AD | < 0.001* |
| AD | CA | 0.001* |
| AD | MT | < 0.001* |
| N | CA | < 0.001§ |
| N | MT | < 0.001§ |
| CA | MT | 0.372§ |
N, normal mucosa (n = 72); AD, adenoma (n = 217); CA, primary colorectal adenocarcinoma (n = 72); MT, metastatic colorectal adenocarcinoma (n = 72).
Mann-Whitney non-parametric test for 2-independent samples.
Wilcoxon signed ranks test for paired samples.
Figure 1.
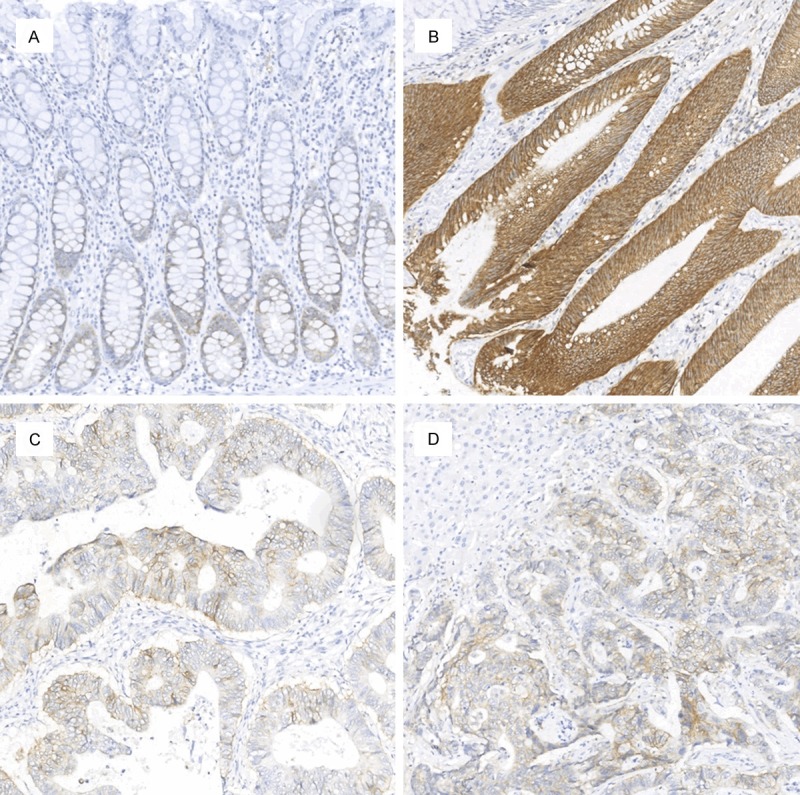
Expression of CD44v6 in the normal, adenoma, primary carcinoma, and liver metastasis sequence. CD44v6 immunoreactivity was minimal in normal tissue (A), maximal in adenoma (B), and moderate in primary carcinoma (C) and liver metastasis (D). There was no difference between primary carcinoma and liver metastasis (magnification, 10×).
Correlation between CD44v6 expression and clinicopathological characteristics
The correlation between CD44v6 expression and the clinicopathological characteristics is shown in Table 3. No statistical associations were found between CD44v6 expression and the different clinicopathological factors analyzed by Pearson’s χ2 test. However, bivariate independent correlation analysis demonstrated an inverse relationship between CD44v6 expression and lymph node metastasis (P = 0.005), as well as perineural invasion (P = 0.036).
Table 3.
Associations between CD44v6 expression and clinicopathological parameters in CRC patients
| CD44v6 expression (n = 402) | |||
|---|---|---|---|
|
|
|||
| Features | Negative n | Positive n | P |
| Age | |||
| < 60 | 42 | 105 | 0.680 |
| ≥ 60 | 68 | 187 | |
| Sex | |||
| Male | 64 | 155 | 0.360 |
| Female | 46 | 137 | |
| Location | |||
| Colon | 58 | 151 | 0.856 |
| Rectum | 52 | 141 | |
| Histotype | |||
| Tubular | 94 | 242 | 0.534 |
| Mucinous, signet ring and undifferentiated | 16 | 50 | |
| pT stage | |||
| pT1-2 | 20 | 60 | 0.596 |
| pT3-4 | 90 | 232 | |
| pN stage | |||
| pN0 | 52 | 163 | 0.125 |
| pN1-2 | 58 | 129 | |
| pM stage | |||
| pM0 | 100 | 264 | 0.879 |
| pM1 | 10 | 28 | |
| Vascular invasion | |||
| Negative | 89 | 255 | 0.102 |
| Positive | 21 | 27 | |
| Perineural invasion | |||
| Negative | 66 | 201 | 0.094 |
| Positive | 44 | 91 | |
| Tumor budding | |||
| Negative | 90 | 226 | 0.335 |
| Positive | 20 | 66 | |
Impact of clinicopathological characteristics and CD44v6 expression on the prognosis of CRC
Univariate survival analyses were used to investigate the prognostic value of clinicopathological features and CD44v6 expression on DSS (Table 4). The results showed that depth of infiltration, lymph node metastasis, distant metastasis, perineural invasion, and tumor budding were statistically associated with DSS. Patients with negative CD44v6 expression tended to have an unfavorable prognosis compared with those with positive expression, although the difference was not statistically significant (Figure 2, P = 0.102). All variables that performed prognostic significance in the univariate analysis were entered into the multivariate survival analysis; CD44v6 expression was also included because of the borderline significant P value. This study identified CD44v6 down-regulation as an independent prognostic factor for a strikingly worse DSS (P = 0.019), along with depth of infiltration, distant metastasis, perineural invasion, and tumor budding (Table 4).
Table 4.
Univariate and multivariate analyses of clinicopathological factors in CRC patients with respect to DSS
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | ||||||
| < 60 | 1 | |||||
| ≥ 60 | 0.093 | 0.635-1.491 | 0.901 | |||
| Sex | ||||||
| Male | 1 | |||||
| Female | 1.017 | 0.707-1.620 | 0.747 | |||
| Location | ||||||
| Colon | 1 | |||||
| Rectum | 0.919 | 0.607-1.391 | 0.690 | |||
| Histotype | ||||||
| Tubular | 1 | |||||
| Mucinous, signet ring and undifferentiated | 0.853 | 0.622-1.169 | 0.322 | |||
| pT stage | ||||||
| pT1-2 | 1 | |||||
| pT3-4 | 3.448 | 1.593-7.461 | 0.002 | 2.764 | 1.237-6.177 | 0.013 |
| pN stage | ||||||
| pN0 | 1 | |||||
| pN1-2 | 2.192 | 1.427-3.366 | < 0.001 | 1.129 | 0.694-1.837 | 0.625 |
| pM stage | ||||||
| pM0 | 1 | |||||
| pM1 | 4.154 | 2.545-6.783 | < 0.001 | 3.864 | 2.321-6.431 | < 0.001 |
| Vascular invasion | ||||||
| Negative | 1 | |||||
| Positive | 1.839 | 1.119-3.024 | 0.016 | 1.154 | 0.684-1.947 | 0.592 |
| Perineural invasion | ||||||
| Negative | 1 | |||||
| Positive | 2.517 | 1.663-3.809 | < 0.001 | 1.584 | 1.012-2.480 | 0.044 |
| Tumor budding | ||||||
| Negative | 1 | |||||
| Positive | 3.081 | 2.019-4.701 | < 0.001 | 2.354 | 1.486-3.728 | < 0.001 |
| CD44v6 expression | ||||||
| Negative | 1 | |||||
| Positive | 0.700 | 0.455-1.076 | 0.104* | 0.586 | 0.375-0.916 | 0.019 |
HR, hazard ratio; CI, confidence interval; P values in bold, statistically significant.
P, borderline significant.
Figure 2.
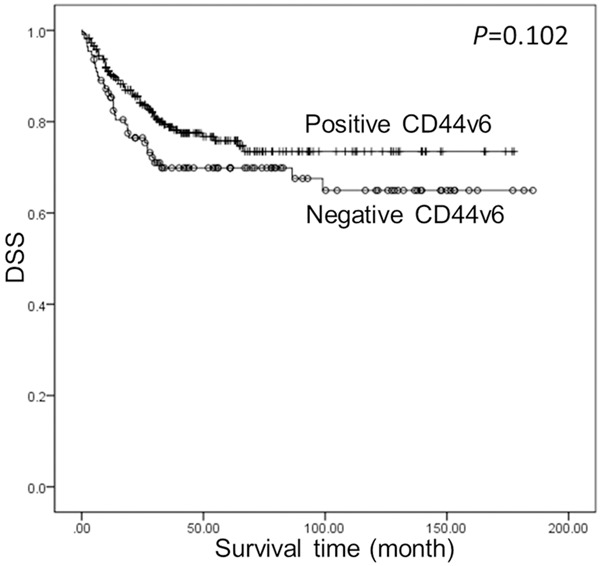
Kaplan-Meier analysis for DSS according to CD44v6 expression. Patients with negative CD44v6 expression tended to have a unfavorable prognosis compared with those with positive expression, although the difference was not statistically significant.
Correlation between CD44v6 and E-cadherin
Spearman’s rank correlation analysis indicated a positive relationship between CD44v6 and E-cadherin (P = 0.034, Rs = 0.011; Figure 3).
Figure 3.
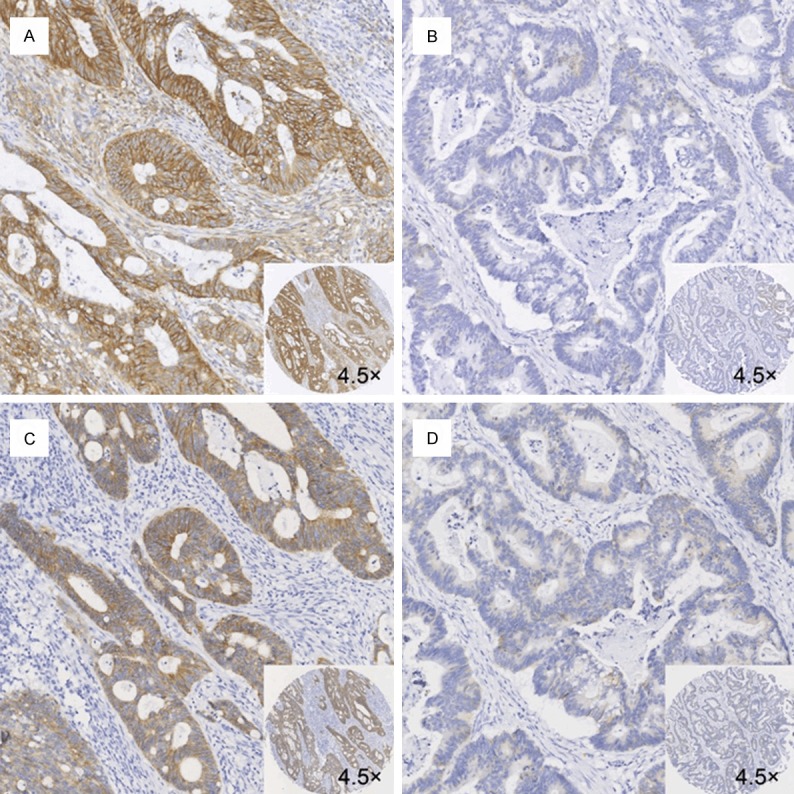
Immunohistochemical analysis of CD44v6 and E-cadherin (membranous) expression in CRC tissues. Positive and corresponding negative staining for CD44v6 (A and B) and E-cadherin (C and D) (magnification, 10×). (A and C) is the same tissue, (B and D) is the same tissue.
CD44v6 expression in the tumor center and invasion front
In the cohort of 402 primary carcinomas, loss of CD44v6 expression was found from the center of the tumor to the invasive front (Z = -3.341, P = 0.001, two-paired sample, Wilcoxon signed ranks test). In the cohort of 72 matched primary carcinomas/metastases, CD44v6 expression also decreased from the tumor center to the invasive front (Z = -3.566, P < 0.001; Figure 4). There was no difference between the center and border in the corresponding liver metastasis (Z = -1.066, P = 0.287).
Figure 4.
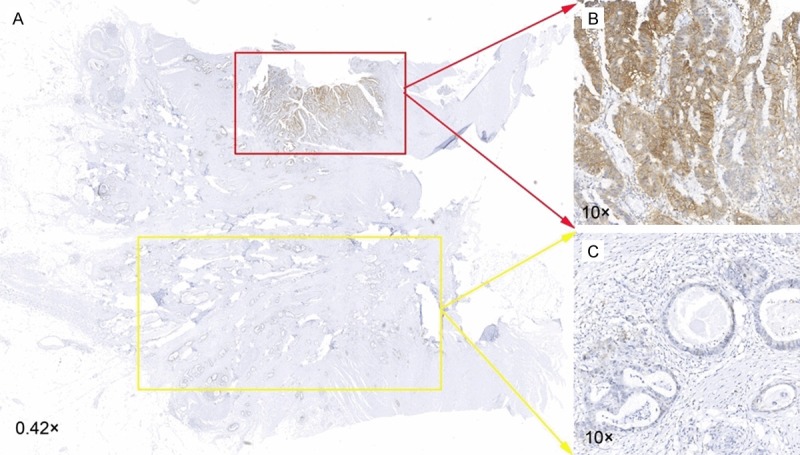
Immunohistochemical analysis of CD44v6 expression in the center and the invasive front ofCRC tissues. A. Whole-tissue scan (NanoZoomer Digital Pathology Scan, Hamamatsu, Japan) (magnification, 0.42×). B. Higher CD44v6 expression in the center of the tissue (magnification, 10×). C. Lower CD44v6 expression in the invasion front of the tissue (magnification, 10×).
Discussion
At present, the contribution of CD44v6 to CRC is disputed. Many studies showed that overexpression of CD44v6 indicated a worse clinical outcome, while other studies suggested its down-regulation being a marker for worse prognosis. In order to understand the function of CD44v6 expression in CRC, TMA combined with whole tissues were used in our study, as well as the adenoma-primary carcinoma-liver metastasis assay.
In our study, we assessed the relationship between CD44v6 expression and relevant clinical parameters of CRC, and the value of CD44v6 for the prognosis of CRC. Our data indicated that loss of CD44v6 is associated with a more aggressive tumor phenotype, including worse survival, perineural invasion, and lymph node metastasis. And multivariate analysis suggested that CD44v6 is an independent prognostic indicator. Some studies support our findings; Chen et al. [13] noted that patients with negative CD44v6 expression had a worse clinical outcome than those with positive expression in stage I/II CRC. Although CD44v6 expression was not showed serving as an independent prognosis indicator, the 5-year disease-specific survival rate for patients with negative CD44v6 expression was significantly lower than those with positive expression [14]. Similar results were observed in other types of aggressive carcinoma, including urothelial bladder cancer [29], lung adenocarcinoma [30], and prostate cancer [31]. All together, these studies suggest that decreased CD44v6 expression is linked to a poorer clinical outcome in CRC.
Interestingly, despite the fact that upregulation of CD44v6 expression was associated with a favorable prognosis; the overall expression of CD44v6 in CRC was higher than that in normal mucosa. By studying the serial process of normal-adenoma-primary carcinoma-liver metastasis tumor progression, we assumed that the difference between colorectal carcinogenesis and progression led to this phenomenon.
In the sequence, CD44v6 immunoreactivity was maximal in adenoma, moderate in primary carcinoma and liver metastasis, and minimal in normal tissue. There was no difference between primary carcinoma and liver metastasis. Similar to our findings, Coppola et al. [15] found that CD44v6 was predominant in adenoma over primary carcinoma and liver metastasis. Contrary to our results, some researchers [23,25] demonstrated that CD44v6 expression is constant throughout the multiple steps of tumor progression. Both Orzechowski et al. [24] and Weg-Remers et al. [26] considered that CD44v6 expression did not differ between adenoma and primary carcinoma, but was significantly decreased in liver metastasis. In addition, Kim et al. [32] found CD44 expression prior to K-ras mutations in colorectal adenoma. CD44 expression is activated by the oncogene ras in cloned rat embryonic fibroblasts [33] and intestinal epithelial cells [34], indicating that CD44 expression is an early event. Kerr et al. [35] reported that CD44v6 staining is higher in atypical adenomatous hyperplasia (AAH) than that in invasive pulmonary adenocarcinoma. Similar to colorectal adenoma, AAH is regarded as a precursor lesion, which plays an essential role in the progression of pulmonary adenocarcinoma. In any case, significant upregulation of CD44v6 occurs in early adenomas, and may play a crucial role in human colorectal carcinogenesis.
Our analyses showed that CD44v6 expression was lower in the invasion front of the CRC than that in the center, which was in line with many studies [14,15,36]. The prognostic effect of CD44v6 downregulation with a poor prognosis in CRC may be due to these results. As a cell adhesion molecule, CD44v6 was significantly associated with E-cadherin expression in our study. And loss of E-cadherin has been described as a predictive factor for an unfavorable prognosis in CRC patients [37]. It is easy for tumor cells to detach from tumors, and gain the ability to invade and metastasize. In our investigation, down-regulation of CD44v6 was associated with lymph node metastasis and perineural invasion. A positive relationship between E-cadherin and CD44v6 has also been reported by Zlobec et al. [14]. In addition, E-cadherin is positively correlated with CD44 in colon cancer [38]. Other than its intercellular adhesion properties, CD44v6 is reported to have a high affinity for hyaluronate [39], which is an important component of the extracellular matrix (ECM). The loss of CD44v6 expression in the front of the tumor leads to defective binding of the tumor cells to the ECM, increasing the metastatic ability [40]. The hypermethylation of the 5’ CpG island of the CD44 gene may be another cause of decreased expression of CD44v6 in human CRCs [41].
A number of studies concerning metastasis from CRC have reported that down-regulation of CD44v6 occurs in the metastatic stage [15,26,42]. Contrary to these reports, our and other studies [14,23,25] found no difference between primary and matched metastatic tumors in CD44v6 expression. Probably, like the case of E-cadherin, re-expression of CD44v6 at the metastatic site may occur during the process of mesenchymal-epithelial transition when metastatic seeding happened [43]. Loss and gain of adhesive functions of the tumor cells may play an important role in the progression of metastatic tumors [44].
The differences in the previous studies are probably due to differences in the primary antibodies used, statistical methods, and study populations. The number of samples is very important, relatively few may lead to a mistaken outcome. The large number of patient specimens in our study could eliminate such potential problems. Combining the TMA with whole tissues strengthens the findings over those obtained by applying these methods alone.
In summary, CD44v6 is upregulated in adenoma, suggesting that the expression of CD44v6 might be an early event in the course of colorectal carcinogenesis which is similar with the genetic mutation of the APC or K-ras genes that have been acknowledged in the colorectal adenoma-carcinoma progress. Once the carcinoma forms in the colorectum, loss of CD44v6 expression at the cell surface of the tumor edge would enhance the progression of metastasis. CD44v6 might play different roles in regulating cell growth and migration. There may be no relationship between metastatic ability and proliferative activity in CRC [45]. So far, no final conclusion has been reached on the role of CD44v6 in CRC. Caution is necessary when considering CD44v6 as a diagnostic and prognostic marker, or as a target for therapeutic strategies in CRC. Further studies with a systematic study including in vivo and in vitro methods are needed to provide definite answers.
Acknowledgements
We thank Dr. Iain Bruce for critical reading of the manuscript. This study was supported by the grants from the National Natural Science Foundation of China (81090420/810090421), Key Science & Technology special project of Zhejiang Province (2012C13014-3), and 111 project (B13026).
Disclosure of conflict of interest
None.
References
- 1.Screaton GR, Bell MV, Bell JI, Jackson DG. The identification of a new alternative exon with highly restricted tissue expression in transcripts encoding the mouse Pgp-1 (CD44) homing receptor. Comparison of all 10 variable exons between mouse, human, and rat. J Biol Chem. 1993;268:12235–8. [PubMed] [Google Scholar]
- 2.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992;89:12160–4. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wielenga VJ, van der Voort R, Mulder JW, Kruyt PM, Weidema WF, Oosting J, Seldenrijk CA, van Krimpen C, Offerhaus GJ, Pals ST. CD44 splice variants as prognostic markers in colorectal cancer. Scand J Gastroenterol. 1998;33:82–7. doi: 10.1080/00365529850166257. [DOI] [PubMed] [Google Scholar]
- 4.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Expression of CD44 and variant proteins in human colorectal cancer and its relevance for prognosis. Scand J Gastroenterol. 1998;33:301–9. doi: 10.1080/00365529850170900. [DOI] [PubMed] [Google Scholar]
- 5.Vizoso FJ, Fernandez JC, Corte MD, Bongera M, Gava R, Allende MT, Garcia-Muniz JL, Garcia-Moran M. Expression and clinical significance of CD44V5 and CD44V6 in resectable colorectal cancer. J Cancer Res Clin Oncol. 2004;130:679–86. doi: 10.1007/s00432-004-0596-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amirghofran Z, Jalali SA, Hosseini SV, Vasei M, Sabayan B, Ghaderi A. Evaluation of CD44 and CD44v6 in colorectal carcinoma patients: soluble forms in relation to tumor tissue expression and metastasis. J Gastrointest Cancer. 2008;39:73–8. doi: 10.1007/s12029-009-9062-2. [DOI] [PubMed] [Google Scholar]
- 7.Peng J, Lu JJ, Zhu J, Xu Y, Lu H, Lian P, Cai G, Cai S. Prediction of treatment outcome by CD44v6 after total mesorectal excision in locally advanced rectal cancer. Cancer J. 2008;14:54–61. doi: 10.1097/PPO.0b013e3181629a67. [DOI] [PubMed] [Google Scholar]
- 8.Huh JW, Kim HR, Kim YJ, Lee JH, Park YS, Cho SH, Joo JK. Expression of standard CD44 in human colorectal carcinoma: association with prognosis. Pathol Int. 2009;59:241–6. doi: 10.1111/j.1440-1827.2009.02357.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhao LH, Lin QL, Wei J, Huai YL, Wang KJ, Yan HY. CD44v6 expression in patients with stage II or stage III sporadic colorectal cancer is superior to CD44 expression for predicting progression. Int J Clin Exp Pathol. 2015;8:692–701. [PMC free article] [PubMed] [Google Scholar]
- 10.Nanashima A, Yamaguchi H, Matsuo S, Sumida Y, Tsuji T, Sawai T, Yasutake T, Nakagoe T, Ayabe H. Expression of multidrug resistance protein in metastatic colorectal carcinomas. J Gastroenterol. 1999;34:582–8. doi: 10.1007/s005350050376. [DOI] [PubMed] [Google Scholar]
- 11.Nanashima A, Yamaguchi H, Sawai T, Yamaguchi E, Kidogawa H, Matsuo S, Yasutake T, Tsuji T, Jibiki M, Nakagoe T, Ayabe H. Prognostic factors in hepatic metastases of colorectal carcinoma: immunohistochemical analysis of tumor biological factors. Dig Dis Sci. 2001;46:1623–8. doi: 10.1023/a:1010680815954. [DOI] [PubMed] [Google Scholar]
- 12.Bendardaf R, Lamlum H, Ristamaki R, Pyrhonen S. CD44 variant 6 expression predicts response to treatment in advanced colorectal cancer. Oncol Rep. 2004;11:41–5. doi: 10.3892/or.11.1.41. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Jiang B, Wang Z, Liu M, Yang H, Xing J, Zhang C, Yao Z, Zhang N, Cui M, Su X. Combined preoperative CEA and CD44v6 improves prognostic value in patients with stage I and stage II colorectal cancer. Clin Transl Oncol. 2014;16:285–92. doi: 10.1007/s12094-013-1069-2. [DOI] [PubMed] [Google Scholar]
- 14.Zlobec I, Gunthert U, Tornillo L, Iezzi G, Baumhoer D, Terracciano L, Lugli A. Systematic assessment of the prognostic impact of membranous CD44v6 protein expression in colorectal cancer. Histopathology. 2009;55:564–75. doi: 10.1111/j.1365-2559.2009.03421.x. [DOI] [PubMed] [Google Scholar]
- 15.Coppola D, Hyacinthe M, Fu L, Cantor AB, Karl R, Marcet J, Cooper DL, Nicosia SV, Cooper HS. CD44V6 expression in human colorectal carcinoma. Hum Pathol. 1998;29:627–35. doi: 10.1016/s0046-8177(98)80014-2. [DOI] [PubMed] [Google Scholar]
- 16.Clarke G, Ryan E, O’Keane JC, Crowe J, Mathuna PM. Mortality association of enhanced CD44v6 expression is not mediated through occult lymphatic spread in stage II colorectal cancer. J Gastroenterol Hepatol. 2000;15:1028–31. doi: 10.1046/j.1440-1746.2000.02285.x. [DOI] [PubMed] [Google Scholar]
- 17.Jungling B, Menges M, Goebel R, Wittig BM, Weg-Remers S, Pistorius G, Schilling M, Bauer M, Konig J, Zeitz M, Stallmach A. Expression of CD44v6 has no prognostic value in patients with colorectal cancer. Z Gastroenterol. 2002;40:229–33. doi: 10.1055/s-2002-25152. [DOI] [PubMed] [Google Scholar]
- 18.Morrin M, Delaney PV. CD44v6 is not relevant in colorectal tumour progression. Int J Colorectal Dis. 2002;17:30–6. doi: 10.1007/s003840100335. [DOI] [PubMed] [Google Scholar]
- 19.Gunther K, Dworak O, Remke S, Pfluger R, Merkel S, Hohenberger W, Reymond MA. Prediction of distant metastases after curative surgery for rectal cancer. J Surg Res. 2002;103:68–78. doi: 10.1006/jsre.2001.6312. [DOI] [PubMed] [Google Scholar]
- 20.Köbel M, Weichert W, Crüwell K, Schmitt WD, Lautenschläger C, Hauptmann S. Epithelial hyaluronic acid and CD44v6 are mutually involved in invasion of colorectal adenocarcinomas and linked to patient prognosis. Virchows Arch. 2004;445:456–64. doi: 10.1007/s00428-004-1095-0. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn S, Koch M, Nubel T, Ladwein M, Antolovic D, Klingbeil P, Hildebrand D, Moldenhauer G, Langbein L, Franke WW, Weitz J, Zoller M. A complex of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins promotes colorectal cancer progression. Mol Cancer Res. 2007;5:553–67. doi: 10.1158/1541-7786.MCR-06-0384. [DOI] [PubMed] [Google Scholar]
- 22.Rao G, Wang H, Li B, Huang L, Xue D, Wang X, Jin H, Wang J, Zhu Y, Lu Y, Du L, Chen Q. Reciprocal interactions between tumor-associated macrophages and CD44-positive cancer cells via osteopontin/CD44 promote tumorigenicity in colorectal cancer. Clin Cancer Res. 2013;19:785–97. doi: 10.1158/1078-0432.CCR-12-2788. [DOI] [PubMed] [Google Scholar]
- 23.Gotley DC, Fawcett J, Walsh MD, Reeder JA, Simmons DL, Antalis TM. Alternatively spliced variants of the cell adhesion molecule CD44 and tumour progression in colorectal cancer. Br J Cancer. 1996;74:342–51. doi: 10.1038/bjc.1996.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orzechowski HD, Beckenbach C, Herbst H, Stolzel U, Riecken EO, Stallmach A. Expression of CD44v6 is associated with cellular dysplasia in colorectal epithelial cells. Eur J Cancer. 1995;31A:2073–9. doi: 10.1016/0959-8049(95)00452-1. [DOI] [PubMed] [Google Scholar]
- 25.Imazeki F, Yokosuka O, Yamaguchi T, Ohto M, Isono K, Omata M. Expression of variant CD44- messenger RNA in colorectal adenocarcinomas and adenomatous polyps in humans. Gastroenterology. 1996;110:362–8. doi: 10.1053/gast.1996.v110.pm8566581. [DOI] [PubMed] [Google Scholar]
- 26.Weg-Remers S, Anders M, von Lampe B, Riecken EO, Schuder G, Feifel G, Zeitz M, Stallmach A. Decreased expression of CD44 splicing variants in advanced colorectal carcinomas. Eur J Cancer. 1998;34:1607–11. doi: 10.1016/s0959-8049(98)00177-4. [DOI] [PubMed] [Google Scholar]
- 27.Xu F, Xu J, Lou Z, Di M, Wang F, Hu H, Lai M. Micropapillary component in colorectal carcinoma is associated with lymph node metastasis in T1 and T2 Stages and decreased survival time in TNM stages I and II. Am J Surg Pathol. 2009;33:1287–92. doi: 10.1097/PAS.0b013e3181a5387b. [DOI] [PubMed] [Google Scholar]
- 28.Morodomi T, Isomoto H, Shirouzu K, Kakegawa K, Irie K, Morimatsu M. An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer. 1989;63:539–43. doi: 10.1002/1097-0142(19890201)63:3<539::aid-cncr2820630323>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.Klatte T, Seligson DB, Rao JY, Yu H, de Martino M, Garraway I, Wong SG, Belldegrun AS, Pantuck AJ. Absent CD44v6 expression is an independent predictor of poor urothelial bladder cancer outcome. J Urol. 2010;183:2403–8. doi: 10.1016/j.juro.2010.01.064. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki H, Yamashiro K. Reduced expression of CD44 v3 and v6 is related to invasion in lung adenocarcinoma. Lung Cancer. 2002;38:137–41. doi: 10.1016/s0169-5002(02)00176-9. [DOI] [PubMed] [Google Scholar]
- 31.Aaltomaa S, Lipponen P, Ala-Opas M, Kosma VM. Expression and prognostic value of CD44 standard and variant v3 and v6 isoforms in prostate cancer. Eur Urol. 2001;39:138–44. doi: 10.1159/000052428. [DOI] [PubMed] [Google Scholar]
- 32.Kim H, Yang XL, Rosada C, Hamilton SR, August JT. CD44 expression in colorectal adenomas is an early event occurring prior to K-ras and p53 gene mutation. Arch Biochem Biophys. 1994;310:504–7. doi: 10.1006/abbi.1994.1199. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann M, Rudy W, Gunthert U, Zimmer SG, Zawadzki V, Zoller M, Lichtner RB, Herrlich P, Ponta H. A link between ras and metastatic behavior of tumor cells: ras induces CD44 promoter activity and leads to low-level expression of metastasis-specific variants of CD44 in CREF cells. Cancer Res. 1993;53:1516–21. [PubMed] [Google Scholar]
- 34.Wimmenauer S, Keller H, Ruckauer KD, Rahner S, Wolff-Vorbeck G, Kirste G, von Kleist S, Farthman EH. Expression of CD44, ICAM-1 and N-CAM in colorectal cancer. Correlation with the tumor stage and the phenotypical characteristics of tumor-infiltrating lymphocytes. Anticancer Res. 1997;17:2395–400. [PubMed] [Google Scholar]
- 35.Kerr KM, MacKenzie SJ, Ramasami S, Murray GI, Fyfe N, Chapman AD, Nicolson MC, King G. Expression of Fhit, cell adhesion molecules and matrix metalloproteinases in atypical adenomatous hyperplasia and pulmonary adenocarcinoma. J Pathol. 2004;203:638–44. doi: 10.1002/path.1557. [DOI] [PubMed] [Google Scholar]
- 36.Avoranta ST, Korkeila EA, Syrjanen KJ, Pyrhonen SO, Sundstrom JT. Lack of CD44 variant 6 expression in rectal cancer invasive front associates with early recurrence. World J Gastroenterol. 2012;18:4549–56. doi: 10.3748/wjg.v18.i33.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zlobec I, Lugli A, Baker K, Roth S, Minoo P, Hayashi S, Terracciano L, Jass JR. Role of APAF-1, E-cadherin and peritumoral lymphocytic infiltration in tumour budding in colorectal cancer. J Pathol. 2007;212:260–8. doi: 10.1002/path.2164. [DOI] [PubMed] [Google Scholar]
- 38.Ngan CY, Yamamoto H, Seshimo I, Ezumi K, Terayama M, Hemmi H, Takemasa I, Ikeda M, Sekimoto M, Monden M. A multivariate analysis of adhesion molecules expression in assessment of colorectal cancer. J Surg Oncol. 2007;95:652–62. doi: 10.1002/jso.20638. [DOI] [PubMed] [Google Scholar]
- 39.Sleeman J, Moll J, Sherman L, Dall P, Pals ST, Ponta H, Herrlich P. The role of CD44 splice variants in human metastatic cancer. Ciba Found Symp. 1995;189:142–51. doi: 10.1002/9780470514719.ch11. discussion 151-6, 174-6. [DOI] [PubMed] [Google Scholar]
- 40.Herrlich P, Morrison H, Sleeman J, Orian-Rousseau V, Konig H, Weg-Remers S, Ponta H. CD44 acts both as a growth- and invasivenesspromoting molecule and as a tumor-suppressing cofactor. Ann N Y Acad Sci. 2000;910:106–18. doi: 10.1111/j.1749-6632.2000.tb06704.x. discussion 18-20. [DOI] [PubMed] [Google Scholar]
- 41.Stallmach A, Wittig BM, Kremp K, Goebel R, Santourlidis S, Zeitz M, Menges M, Raedle J, Zeuzem S, Schulz WA. Downregulation of CD44v6 in colorectal carcinomas is associated with hypermethylation of the CD44 promoter region. Exp Mol Pathol. 2003;74:262–6. doi: 10.1016/s0014-4800(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 42.Bendardaf R, Elzagheid A, Lamlum H, Ristamaki R, Collan Y, Pyrhonen S. E-cadherin, CD44s and CD44v6 correlate with tumour differentiation in colorectal cancer. Oncol Rep. 2005;13:831–5. doi: 10.3892/or.13.5.831. [DOI] [PubMed] [Google Scholar]
- 43.Wells A, Yates C, Shepard CR. E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas. Clin Exp Metastasis. 2008;25:621–8. doi: 10.1007/s10585-008-9167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hart IR, Goode NT, Wilson RE. Molecular aspects of the metastatic cascade. Biochim Biophys Acta. 1989;989:65–84. doi: 10.1016/0304-419x(89)90035-8. [DOI] [PubMed] [Google Scholar]
- 45.Zhang JC, Wang ZR, Cheng YJ, Yang DZ, Shi JS, Liang AL, Liu NN, Wang XM. Expression of proliferating cell nuclear antigen and CD44 variant exon 6 in primary tumors and corresponding lymph node metastases of colorectal carcinoma with Dukes’ stage C or D. World J Gastroenterol. 2003;9:1482–6. doi: 10.3748/wjg.v9.i7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]


