Abstract
Trefoil factor 3 (TFF3), a regulatory protein composed of 59 amino acids, has been suggested to be involved in pathogenesis, proliferation, differentiation, invasion, migration and apoptosis in multiple malignant tumors. This study thus investigated the effect of TFF3 knockout in human pituitary adenoma cell line HP75 on cell apoptosis and related pathways. RNA interference approach was used to knock down the expression of TFF3 protein. The gene silencing was validated by RNA denaturing gel electrophoresis and Western blotting. The effect of TFF3 knockout on cell apoptosis was analyzed by Western blotting and flow cytometry. TFF3 protein level in pituitary adenoma was about 3.61 ± 0.48 folds of that in normal tissues (P < 0.01). After transfecting with small interference RNA (siRNA) against TFF3, the apoptotic ration was significantly elevated (P < 0.01). Apoptosis related protein Bcl-2 and caspase-3 levels were remarkably depressed after siRNA transfection, while Bax and cleaved caspase-3 levels were elevated. TFF3 protein knockout can facilitate apoptosis of human pituitary adenoma HP75 cells via mitochondrial pathway.
Keywords: Trefoil factor 3, pituitary adenoma, HP75 cell, RNA interference, cell apoptosis
Introduction
Pituitary adenoma is formed by monoclonal proliferation of gland cells in anterior pituitary. It is one common intracranial tumor, occupying about 10%~15% of total cases, only next to glioma and meningioma [1-3]. About 3.3% of tumors may invade adjacent tissues including cavernous sinus and hypothalamus, making the surgical resection extremely difficult [4-6]. Those patients, therefore, need radio- or chemo-therapy after surgery, significantly compromising their life qualities. All these factors contribute to relatively higher recurrence rate of pituitary adenoma [2,7-10]. Therefore, pathogenesis and effective treatment pathway of pituitary adenoma have been widely explored but without molecular mechanism fully understood.
Trefoil factor 3 (TFF3) is one regulatory protein composed of 59 amino acid residues. Recent studies have indicated the important role of TFF3 in occurrence, proliferation, differentiation, invasion, migration and apoptosis of multiple human tumors [11,12]. The expression level of TFF3 protein can be used as one indicator for tumor progression [13]. TFF3 knockout can also significantly enhance the sensitivity of tumor cells to radio- or chemo-therapy, beside the inhibition on tumor cell proliferation, invasion and migration [14,15]. In this study, the expression of TFF3 protein in human pituitary adenoma cell line HP75 was silenced by small interference RNA (siRNA), in order to investigate the effect of TFF3 knockdown on cell apoptosis, along with the elucidation of apoptotic regulatory pathway. This study aimed to provide further evidence for clinical treatment of pituitary adenoma using TFF3 as the target.
Materials and methods
HP75 cell culture and transfection
Human pituitary adenoma cell HP75 was resuscitated and incubated in low-glucose DMEM complete medium (Gibco, US) containing 10% fetal bovine serum (FBS, Gibco, US) in a humidified chamber with 5% CO2 at 37°C. Until reaching confluence of 80%~90%, cells were digested in 0.25% trypsin containing EDTA for passage.
Before transfection, cells were seeded into 48-well plate at 6 × 105/mL and were divided into negative control (transfecting with controlled siRNA vector) and experimental group (transfected with siTFF3-siRNA vectors). 18 hours after transfection, supernatants containing viral vectors were removed. Cells were re-suspended in 2 mL RPMI medium containing 10% FBS. After 48 hours, images were taken to deduce the transfection efficiency.
Clinical sample collection
A total of 24 tissues samples were collected from pituitary adenoma surgeries from August 2013 to May 2015 in the department of neurosurgery of XXX hospital. Among all 24 patients, there were 13 males and 11 females, aging between 55 and 72 years old (average age = 65.4 years). All cases have confirmed diagnosis by pathological examination, and have complete medical records. Tissue samples were rinsed in PBS and kept in liquid nitrogen. This study has been pre-approved by the ethical committee of the host hospital and has obtained written consents from all participants.
Western blotting
Cells were collected and rinsed in cold PBS, and were lysed in 0.2 mL lysis buffer for extracting total proteins. 20 μg proteins were separated by SDS-PAGE, and were transferred onto PVDF membrane. The membrane was incubated with primary antibody against Bcl-2, GAPDH, TFF3, Bax or GAPDH (Abcam, US). Secondary antibody including goat anti-mouse IgG IR Dye 800cw and goat anti-rabbit IgG IR Dye 800cw (Odyssey, US) was then applied for continuous incubation. After PBS rinsing, Odyssey scanning system was used to visualize the signal. Protein expression level was calculated against GAPDH.
RNA gel electrophoresis
1.2% formaldehyde-based denaturing agarose was prepared. RNA samples were mixed with 1.0 μL ethidium bromide and were loaded onto agarose gel. Gel electrophoresis was performed under 10 V/cm electrical field for 30 min. Ultraviolet imaging system was used to analyze experiment results.
Cell apoptosis assay
HP75 cells were firstly digested by 0.25% trypsin for preparing single cell suspension. After rinsing in PBS for three times, cell were resuspended at 105/mL concentration. Annexin V-FITC (5 μL) was firstly added, followed by PI dye (5 μL). The mixture was incubated in dark for 30 min, and was subsequently loaded for flow cytometry assay. Each experiment was triplicated (N = 3).
Statistical analysis
All experimental data were analyzed by SPSS 18.0 software, and were presented by mean ± standard deviation (SD). Independent two-sample student t-test was used to compare means. A statistical significance was defined when P < 0.05.
Results
TFF expression in human pituitary adenoma tissues
All 24 samples of human pituitary tissues were identified as invasive pituitary adenoma. Western blotting was performed on tumor and adjacent tissues. Results showed significantly elevated TFF3 protein level in tumor tissue compared to adjacent normal tissue (P < 0.01, Figure 1).
Figure 1.
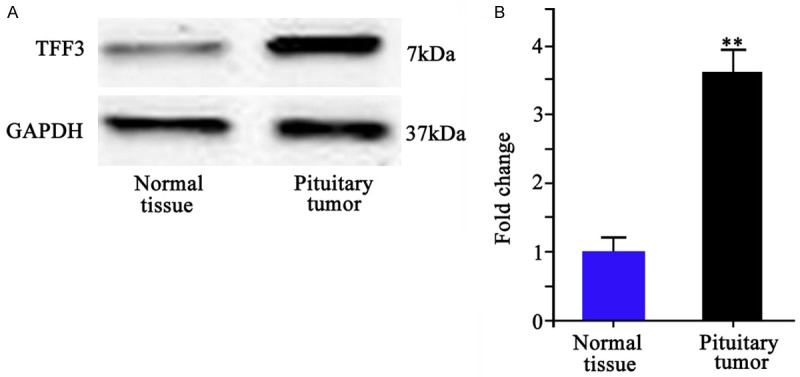
TFF3 protein expression. A. Western blotting bands of TFF3 protein in normal pituitary tissue (left) and in pituitary tumor (right). B. Quantitative data of TFF3 in normal and tumor tissues. **P < 0.01 compared to normal tissue.
TFF3 expression after siRNA transfection
HP75 cells were transferred with controlled siRNA vector or siTFF3-siRNA vector. RNA gel electrophoresis results showed significantly depressed mRNA level in siRNA transfected cells (Figure 2A). Western blotting further showed decreased protein levels (Figure 2B). Therefore efficient siRNA transfection is achieved.
Figure 2.
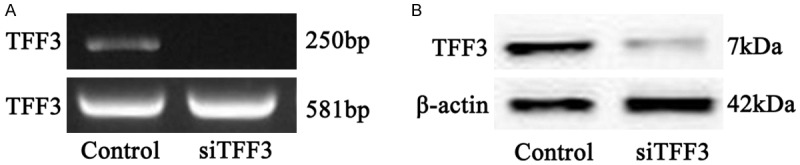
TFF3 expression level in HP75 cells after transfection. A. RNA gel electrophoresis; B. Western blotting bands.
HP75 cell apoptosis after TFF3 protein knockdown
Using annexin V-FITC and PI double labelling, flow cytometry results showed significantly elevated apoptotic level of HP75 cells after TFF3-siRNA transfection, as compared to control group (18.33 ± 1.93 vs. 3.85 ± 0.98, P < 0.01, Figure 3).
Figure 3.
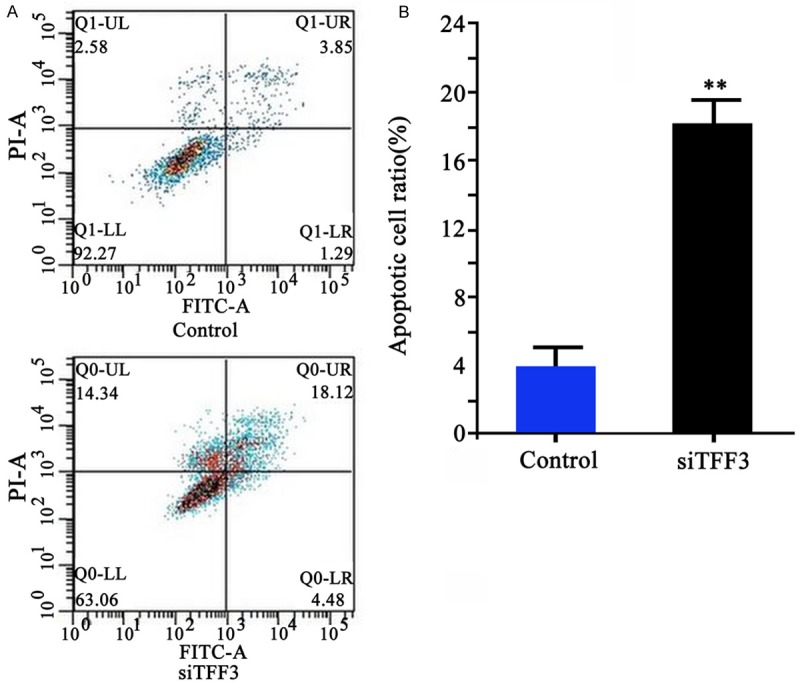
Cell apoptosis of HP75 cells after TFF3 knockdown. A. Flow cytometry results (FITC/PI); B. Quantitative results of cell apoptosis after TFF3 protein knockdown. **P < 0.01 compared to control group.
Apoptotic proteins expression in HP75 cells
48 hours after TFF3 knockdown, Western blotting was performed to detect expressional profile of apoptotic related protein Bcl-2 and Bax. Result showed significantly decreased Bcl-2 protein, and increased pro-apoptotic protein Bax expression levels after TFF3 knockdown (Figure 4, P < 0.01). Further studies showed that, 48 hours after TFF3 knockdown, the precursor of caspase-3 protein, procaspase-3, was significantly down-regulated, with elevated level of proteolytic fragments (Figure 4, P < 0.01). These results collectively suggested the silencing of TFF3 protein facilitated apoptosis of HP75 cells via endogenously mitochondrial pathway.
Figure 4.
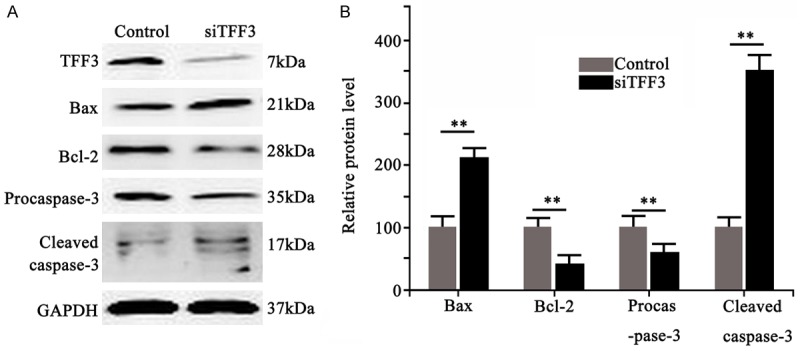
Apoptotic protein levels in HP75 cells after TFF3 silencing. A. Western blotting bands showing cell apoptosis related proteins Bax, Bcl-2, Procaspase-3 and cleaved caspase-3 levels. B. Quantitative results of protein expression level.
Discussion
Recent clinical studies have revealed the pathogenesis of pituitary adenoma including interaction between multiple cytokines and genes. TFF3 is one regulatory protein composed of 59 amino acid residues. Recent studies have indicated the important role of TFF3 in occurrence, proliferation, differentiation, invasion, migration and apoptosis of multiple human tumors. The expression level of TFF3 protein can be used as one indicator for tumor progression [11-13]. TFF3 knockout can also significantly enhance the sensitivity of tumor cells to radio- or chemo-therapy, beside the inhibition on tumor cell proliferation, invasion and migration [14,15]. This study investigated a total of 24 pituitary adenoma samples and found low expression level of TFF3 in normal people, while tumor patients had significantly higher level. Therefore, the role of TFF3 protein in the pathogenesis of pituitary adenoma is of critical importance.
This study firstly down-regulated TFF3 protein expression by RNA interference, and validated by RNA denaturing agarose gel electrophoresis and Western blotting. After knockdown of TFF3 in HP75 cells, the percentage of Annexin V-FITC and PI double positive cells was significantly elevated from 3.85% ± 0.98% to 18.33% ± 1.93%. As apoptosis is the result of a series of interactions between apoptotic related proteins, we further tested apoptosis related proteins including Bcl-2, Bax and caspase-3 proteins in HP75 cells 48 hours after transfection. Our results showed significantly depressed anti-apoptotic protein Bcl-2 and caspase-3 precursors expression after TFF3 knockdown, and elevated pro-apoptotic proteins Bax and caspase-3 cleaved fragment levels.
Bcl-2 is a type of intracellular protein that is coded by oncogene bcl-2 that inhibits cell apoptosis. Its over-expression may induce the malignant clonal proliferation of tumors [16]. Bax protein is one membrane perforated protein that can stimulate cytochrome C release, and can initiate caspase-3 proteinase-induced endogenous apoptotic pathway after activation. The over-expression of pro-apoptotic protein Bax can inhibit tumor cell proliferation in addition to facilitating cell apoptosis [17]. Both belonged to Bcl-2 protein family, Bax and Bcl-2 proteins play an important modulatory role in the means of their relative ratio [18]. In most tumor cells, the anti-apoptotic protein Bcl-2 was up-regulated while pro-apoptotic protein Bax is down-regulated, thus inhibiting tumor cell apoptosis. In contrast, the down-regulation of Bcl-2 and up-regulation of Bax can activate caspase-3 proteinase precursor, procaspase-3, further activating downstream apoptotic signaling pathway. Caspase-3 proteinase plays a crucial role in endogenous (mitochondrial) apoptotic pathway. The activation of caspase-3 proteinase can further induce apoptotic related proteins, for initiating mitochondria-induced endogenous apoptosis, finally leading to the alternation of molecular biological and morphological properties of tumors. Therefore the activation of Bax is regarded as one of key steps in caspase-3 protein activation, which can initiate mitochondria-induced endogenous apoptosis program, further leading to cell death [19]. Our study found significantly depressed expression levels of Bcl-2 and caspase-3 precursor proteins in HP75 cells with TFF3 knockdown, whilst Bax and caspase-3 cleaved fragments were significantly elevated [20]. These data collectively suggested the induced cell apoptosis of HP75 cell line by TFF3 protein knockdown by mitochondrial pathway.
This study showed significantly elevated TFF3 protein expression level in pituitary adenoma patients compared to normal peoples. The knockdown of TFF3 protein expression by siRNA approach was shown to induce tumor cell apoptosis via mitochondrial pathway. Our study provides new insights regarding the precise treatment of pituitary tumor and evaluating biological behavior of tumors. Further exploration of TFF3 may provide novel drug targets for the diagnosis and treatment of pituitary adenoma.
Disclosure of conflict of interest
None.
References
- 1.Fujimoto Y, Balsalobre L, Santos FP, Vellutini E, Stamm AC. Endoscopic combined “transseptal/transnasal” approach for pituitary adenoma: reconstruction of skull base using pedicled nasoseptal flap in 91 consecutive cases. Arq Neuropsiquiatr. 2015;73:611–5. doi: 10.1590/0004-282X20150070. [DOI] [PubMed] [Google Scholar]
- 2.Yilmaz B, Ekşi MŞ, Ekşi EE, Toktaş ZO, Akakın A, Kılıç T. Coexistence of arteriovenous malformation with nonfunctioning pituitary adenoma. J Neurosci Rural Pract. 2015;6:458–60. doi: 10.4103/0976-3147.158762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharifi G, Bakhtevari MH, Alghasi M, Saberi M, Dehghan M, Bidari F, Rezaei O. Hard calcified intrasellar schwannoma mimicking pituitary adenoma: A case report and review of the literature. Clin Neurol Neurosurg. 2015;137:38–43. doi: 10.1016/j.clineuro.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Nie S, Li K, Huang Y, Zhao J, Gao X, Sun J. Endoscopic endonasal transsphenoidal surgery for treating pituitary adenoma via a subseptum mucosa approach. Int J Clin Exp Med. 2015;8:5137–43. [PMC free article] [PubMed] [Google Scholar]
- 5.Raverot G, Assié G, Cotton F, Cogne M, Boulin A, Dherbomez M, Bonneville JF, Massart C. Biological and radiological exploration and management of non-functioning pituitary adenoma. Ann Endocrinol (Paris) 2015;76:201–9. doi: 10.1016/j.ando.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Cortet-Rudelli C, Bonneville JF, Borson-Chazot F, Clavier L, Coche Dequéant B, Desailloud R, Maiter D, Rohmer V, Sadoul JL, Sonnet E, Toussaint P, Chanson P. Post-surgical management of non-functioning pituitary adenoma. Ann Endocrinol (Paris) 2015;76:228–38. doi: 10.1016/j.ando.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Brandstatter W, Hatzl M, Weis S, Javor A, Gabriel M. Successful resection of TSH-secreting pituitary adenoma demonstrated by serial 99mTc-scintigraphy. Nuklearmedizin. 2015;54:N23–4. [PubMed] [Google Scholar]
- 8.Zhan R, Chen S, Xu S, Liu JK, Li X. Postoperative Low-Flow Cerebrospinal Fluid Leak of Endoscopic Endonasal Transsphenoidal Surgery for Pituitary Adenoma--Wait and See, or Lumbar Drain? J Craniofac Surg. 2015;26:1261–4. doi: 10.1097/SCS.0000000000001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCoul ED, Bedrosian JC, Akselrod O, Anand VK, Schwartz TH. Preservation of multidimensional quality of life after endoscopic pituitary adenoma resection. J Neurosurg. 2015;123:813–20. doi: 10.3171/2014.11.JNS14559. [DOI] [PubMed] [Google Scholar]
- 10.Fuminari K, Hideki A, Manabu O, Mitsunori M. Extended endoscopic endonasal surgery using three-dimensional endoscopy in the intra-operative MRI suite for supra-diaphragmatic ectopic pituitary adenoma. Turk Neurosurg. 2015;25:503–7. doi: 10.5137/1019-5149.JTN.11212-14.1. [DOI] [PubMed] [Google Scholar]
- 11.Kannan N, Kang J, Kong X, Tang J, Perry JK, Mohankumar KM, Miller LD, Liu ET, Mertani HC, Zhu T, Grandison PM, Liu DX, Lobie PE. Trefoil factor 3 is oncogenic and mediates anti-estrogen resistance in human mammary carcinoma. Neoplasia. 2010;12:1041–53. doi: 10.1593/neo.10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CW, Shen SC, Hou WC, Yang LY, Chen YC. Heme oxygenase-1 inhibits breast cancer invasion via suppressing the expression of matrix metalloproteinase-9. Mol Cancer Ther. 2008;7:1195–206. doi: 10.1158/1535-7163.MCT-07-2199. [DOI] [PubMed] [Google Scholar]
- 13.Pandey V, Wu ZS, Zhang M, Li R, Zhang J, Zhu T, Lobie PE. Trefoil factor 3 promotes metastatic seeding and predicts poor survival outcome of patients with mammary carcinoma. Breast Cancer Res. 2014;16:429. doi: 10.1186/s13058-014-0429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perera O, Evans A, Pertziger M, MacDonald C, Chen H, Liu DX, Lobie PE, Perry JK. Trefoil factor 3 (TFF3) enhances the oncogenic characteristics of prostate carcinoma cells and reduces sensitivity to ionising radiation. Cancer Lett. 2015;361:104–11. doi: 10.1016/j.canlet.2015.02.051. [DOI] [PubMed] [Google Scholar]
- 15.Pandey V, Jung Y, Kang J, Steiner M, Qian PX, Banerjee A, Mitchell MD, Wu ZS, Zhu T, Liu DX, Lobie PE. Artemin Reduces Sensitivity to Doxorubicin and Paclitaxel in Endometrial Carcinoma Cells through Specific Regulation of CD24. Transl Oncol. 2010;3:218–29. doi: 10.1593/tlo.09325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandey V, Qian PX, Kang J, Perry JK, Mitchell MD, Yin Z, Wu ZS, Liu DX, Zhu T, Lobie PE. Artemin stimulates oncogenicity and invasiveness of human endometrial carcinoma cells. Endocrinology. 2010;151:909–20. doi: 10.1210/en.2009-0979. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Su L, Liu X. Loss of CDH1 up-regulates epidermal growth factor receptor via phosphorylation of YBX1 in non-small cell lung cancer cells. FEBS Lett. 2013;587:3995–4000. doi: 10.1016/j.febslet.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 18.Yan Y X, Li WZ, Huang YQ, Liao WX. The COX-2 inhibitor Celecoxib enhances the sensitivity of KB/VCR oral cancer cell lines to Vincristine by down-regulating P-glycoprotein expression and function. Prostaglandins Other Lipid Mediat. 2012;97:29–35. doi: 10.1016/j.prostaglandins.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Yuan Z, Wang H, Hu Z, Huang Y, Yao F, Sun S, Wu B. Quercetin inhibits proliferation and drug resistance in KB/VCR oral cancer cells and enhances its sensitivity to vincristine. Nutr Cancer. 2015;67:126–36. doi: 10.1080/01635581.2015.965334. [DOI] [PubMed] [Google Scholar]
- 20.Yuan Z, Yao F, Hu Z, Sun S, Wu B. Quercetin inhibits the migration and proliferation of astrocytes in wound healing. Neuroreport. 2015;26:387–93. doi: 10.1097/WNR.0000000000000352. [DOI] [PubMed] [Google Scholar]


