Fig. 4.
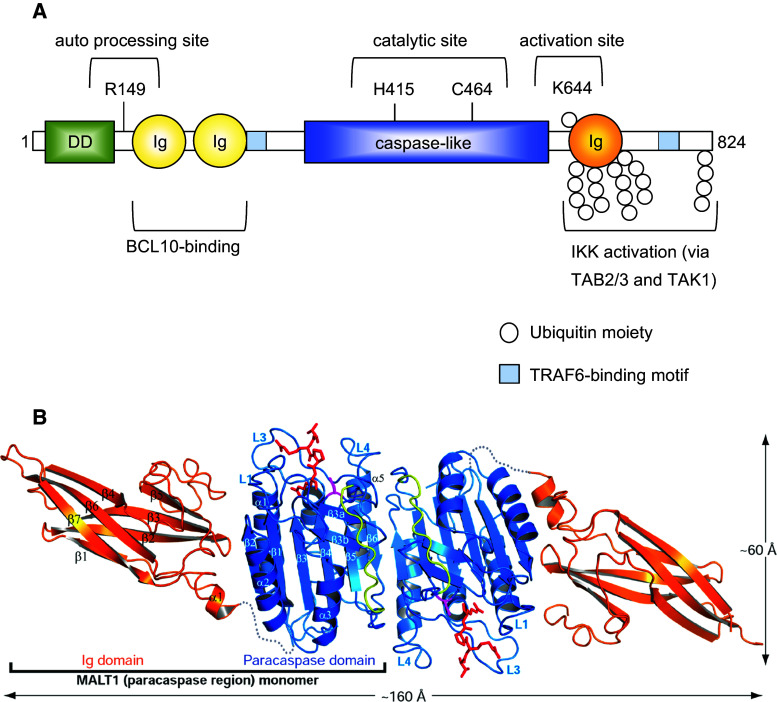
Molecular structure and function of MALT1. a MALT1 contains an N-terminal death domain (DD), followed by two immunoglobulin-like domains (Ig), which are required for the interaction between MALT1 and BCL10. The central caspase-like domain, with the active site residues H415 and C464, is followed by a third Ig domain that contains K644, a monoubiquitination site that controls protease activity. MALT1 contains two binding motifs for the ubiquitin ligase TRAF6 (tumor necrosis factor receptor-associated factor 6). TRAF6 polyubiquitinates MALT1 on multiple C-terminal lysine residues, generating K63-linked ubiquitin chains that can in turn promote activation of the inhibitor of NF-κB kinase (IKK) complex through recruitment of the IKK-activating kinase TAK1 via the adaptor proteins TAB 2/3. Amino acid numbering in the figure refers to human MALT1. b Ribbon representation of the crystallographic structure of the MALT1 homodimer (figure reproduced from Yu et al., PNAS 108 (52), 21004–21009 (2011), with kind permission) [45]. The shown crystallized fragment comprises the paracaspase region (blue) and the adjacent C terminal Ig domain (orange). The covalently bound MALT1 peptide inhibitor is colored red and the subunit linker in the caspase-like fold is colored yellow. The sequences between the caspase-like domain and the Ig domain are disordered and represented as gray dotted lines