Figure 7. Schematic representation of the role of purinergic signalling mechanisms in cell–cell interactions at the rat carotid body ‘tripartite’ synapse .
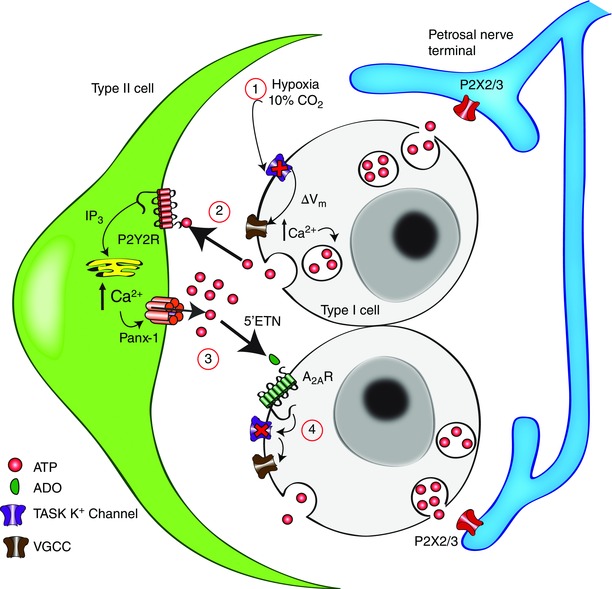
The chemostimuli hypoxia and hypercapnia depolarize type I cells through inhibition of TASK1/3 K+ channels (1), leading to Ca2+ entry through voltage‐gated Ca2+ channels (VGCC) and ATP release (2). ATP stimulates postsynaptic P2X2/3 receptors on petrosal afferent nerve terminals causing excitation, as well as P2Y2 receptors (P2Y2R) on adjacent glial‐like type II cells. P2Y2R stimulation leads to Ca2+ release from intracellular stores via inositol trisphosphate (IP3) signalling pathways and opening of pannexin‐1 channels, allowing further release of ATP into the synaptic cleft. ATP (from both type II and type I cells) is broken down by extracellular 5′‐ectonucleotidase (5′ENT) into adenosine (ADO) (3), which activates primarily ADO A2A receptors (A2AR) on type I cells. Activation of A2A receptors leads to inhibition of TASK1/3 channels to further enhance type I cell depolarization (4) (Xu et al. 2006), and therefore ATP release. Omitted for clarity is the pathway by which hypoxia stimulates ADO release from type I cells via a plasma membrane nucleoside transporter, and negative feedback pathways by which ATP inhibits type I cells via P2Y1 receptors (Xu et al. 2005) and pannexin‐1 channels in type II cells (Nurse, 2014).
