Fig. 4.
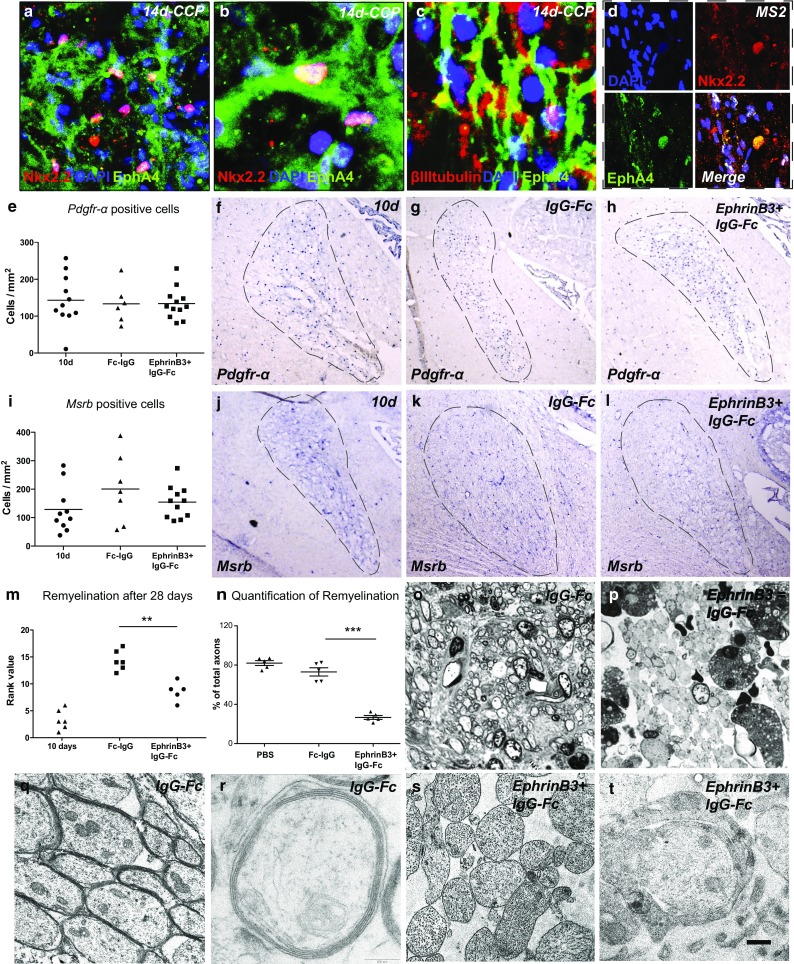
The presence of EphrinB3 inhibits CNS remyelination. a–d EphA4 receptor is expressed by Nkx2.2+ OPCs and βIII-tubulin+ axons in experimental CCP lesions, as well as d in chronic active human white matter MS lesions. e–h In situ hybridization for Pdgfr-α showed comparable OPC densities within lesions of EphrinB3-treated animals and controls 28 days after lesion induction (dpi) (10 dpi—prior to EphrinB3 infusion: n = 11; IgG-Fc: n = 6; EphrinB3 + IgG-Fc: n = 12; ANOVA: P > 0.1). i–l Similarly, levels of Msrb + macrophages remained unaffected (10 dpi: n = 9; IgG-Fc: n = 7; EphrinB3 + IgG-Fc: n = 11; ANOVA: P > 0.1). m–t The extent of remyelination was assessed on methylene blue and Azur-II-stained semithin sections. EphrinB3 infusion induced a significant impairment of CNS remyelination as compared to controls (IgG-Fc control n = 6; EphrinB3 n = 5; Mann–Whitney U test; **P < 0.001). n Quantification of demyelinated and demyelinated axons confirms that the presence of EphrinB3 impairs CNS remyelination (PBS control n = 5, IgG-Fc control n = 6; EphrinB3 n = 5; t test; ***P < 0.0001). o Remyelination in controls was nearly complete at 28 dpi; however, p in EphrinB3-infused animals, the majority of axons remained demyelinated. q, r EM analysis demonstrated successful formation of compact myelin sheaths in controls, whereas s, t in EphrinB3-infused lesions axons remained demyelinated. t Occasionally, axons were contacted by oligodendroglial processes in EphrinB3-treated animals that failed to form compact myelin. Error bars ± SEM. Scale bar in a = 30 μm; in b, c = 50 μm; in d = 40 μm; in f–h, j–l = 200 μm; in o, p = 100 μm; in q, r = 2 μm; in s, t = 0.5 μm
