Figure 5.
Synergistic Cytotoxic Effect in U2OS Cancer Cells by Combination Treatment of AZD7762/VE-821 Is Mainly Due to CDK-Mediated Excess Origin Firing
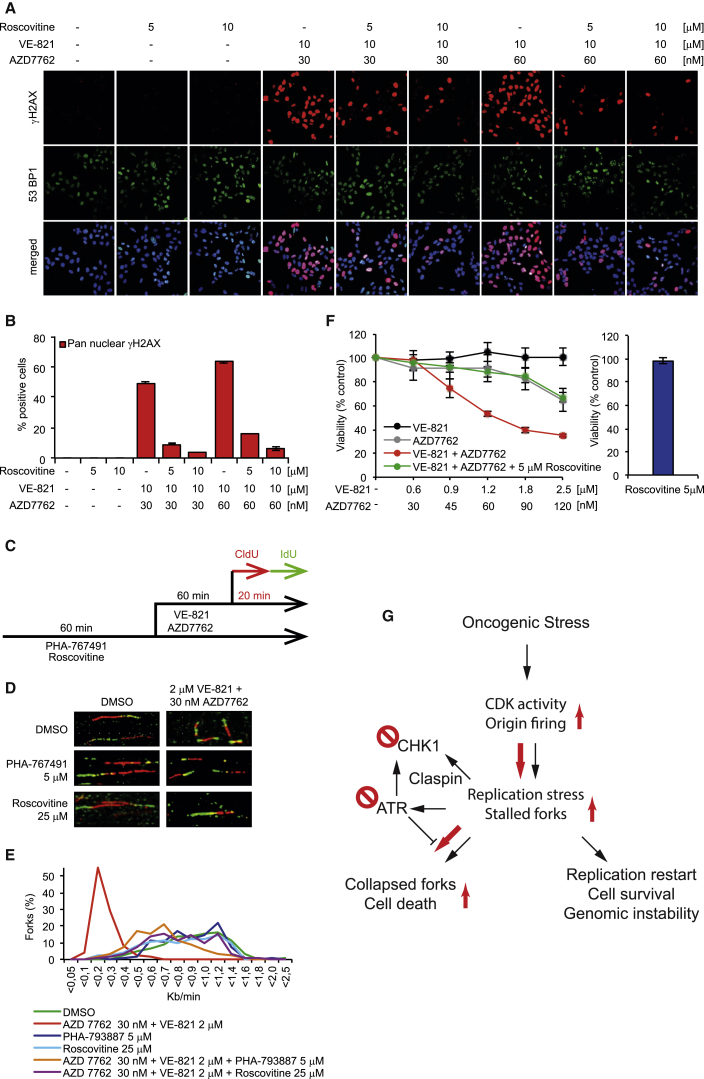
(A) U2OS cells were pretreated with the indicated concentrations of the CDK inhibitor Roscovitine for 1 hr prior to the addition of VE-821 and AZD7762 for 24 hr. Cells were probed with anti-phospho (Serine 139) H2AX antibody and anti-53BP1, and DNA was counterstained with ToPro.
(B) Quantitative data of pan-nuclear γH2AX are shown (mean ± SEM from two independent experiments).
(C) Treatment regimen for DNA fiber assay in U2OS cells is shown.
(D) CDK inhibitors Roscovitine and PHA-767491 enhance the replication fork speed of U2OS cells treated with VE-821 and AZD7762 in combination. U2OS cells were pre-treated with Roscovitine or PHA-767491 for 1 hr prior to the addition of VE-821 and AZD7762. Representative images show stained replication fork tracts for each treatment group.
(E) Average distribution of replication fork rates. A minimum of 450 forks per condition were analyzed from at least three independent repeats.
(F) Roscovitine abolishes the synergistic cytotoxic effect of combination treatment of AZD7762 and VE-821 in U2OS cancer cells. U2OS cells were individually treated with VE-821, AZD7762, or the combination with our without Roscovitine for 24 hr, followed by recovery for another 48 hr. Cell viability was measured by using resazurin at 72 hr.
(G) Model for ATR/CHK1 synthetic lethality. CHK1 is activated by replication stress both by ATR-dependent and -independent pathways (Yang et al., 2008) to suppress replication stress in cancer, promoting restart and survival. CHK1 inhibitors increase oncogene-activated CDK activity and origin firing, leading to replication stress and accumulation of stalled replication forks, requiring ATR activity to prevent replication collapse. Red arrows indicate primary route in the presence of both ATR and CHK1 inhibitors.