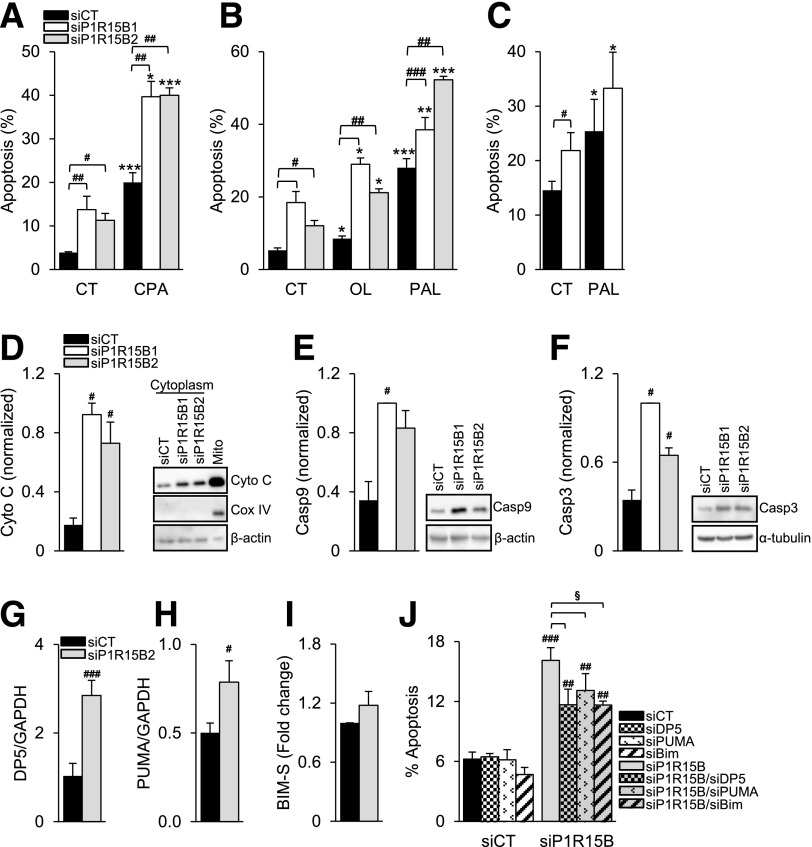
Figure 6.
PPP1R15B deficiency sensitizes β-cells to FFA- and ER stress–induced apoptosis and activates the intrinsic pathway of apoptosis via DP5, PUMA, and Bim-S. INS-1E (A and B) or primary rat β-cells (C) were transfected with a control siRNA (siCT) or two different siRNAs targeting PPP1R15B (siP1R15B1 and siP1R15B2). After a 24-h transfection, the cells were exposed or not (CT) to CPA, oleate (OL), or palmitate (PAL) for 16 (A and B) or 24 h (C) (n = 4–5). D: Mitochondrial cytochrome c (Cyto C) release was detected by Western blot in the cytoplasmic fraction 48 h after PPP1R15B knockdown. The right lane shows a noncytoplasmic fraction that includes mitochondria (Mito). Cox IV was used as a mitochondrial control and β-actin as a cytoplasmic control. Activation of caspase-9 (Casp9) (E) and caspase-3 (Casp3) (F) was detected by Western blot 48 h after PPP1R15B knockdown. β-Actin and α-tubulin were used as loading controls. D, E, and F are representative blots of 4–5 experiments. The densitometry data were normalized to the highest value. DP5 (G) and PUMA (H) mRNA expression were measured by real-time PCR and corrected for the reference gene GAPDH (n = 4). Bim-S levels were measured by Western blot (Supplementary Fig. 2), corrected for α-tubulin, and expressed as fold of siCT (I) (n = 5). J: PPP1R15B was silenced alone or in combination with DP5, PUMA, or Bim, and apoptosis was examined by Hoechst 33342/propidium iodide staining (n = 3–4). *P < 0.05, **P < 0.01, ***P < 0.001, treated vs. control. #P < 0.05, ##P < 0.01, ###P < 0.001, siP1R15B vs. siCT. §P < 0.05, single vs. double knockdown.